��Ŀ����
����Ŀ���״���һ����Ҫ�Ļ���ԭ�ϣ�����һ�ֿ�������Դ�����п�����Ӧ�õĹ���ǰ����
(1)��֪��CH3OH(g)===HCHO(g)��H2(g) ��H����84 kJ��mol��1
2H2(g)��O2(g)===2H2O(g) ��H����484 kJ��mol��1
�ٹ�ҵ�ϳ��Լ״�Ϊԭ����ȡ��ȩ����д��CH3OH(g)��O2(g)��Ӧ����HCHO(g)��H2O(g)���Ȼ�ѧ����ʽ��______________________________________________��
���������Ʊ���ȩʱ������Ӧ����ͨ���ʵ���������������Ŀ��_________________��
(2)��ҵ�Ͽ������·����ϳɼ״�����ѧ����ʽΪCO(g)��2H2(g)==CH3OH(g)����֪ijЩ��ѧ���ļ����������±���
��ѧ�� | C��C | C��H | H��H | C��O | C��O | O��H |
����/(kJ��mol��1) | 348 | 413 | 436 | 358 | x | 463 |
��ش��������⣺
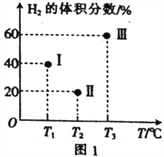
��ͼ1������a������b�Ĵ�ʩ��_________��

����֪CO�е�C��O֮��Ϊ�����������Ϊx kJ/mol����x��________��
(3)�ɼ״���������NaOH��Һ���ɵ������ֻ���أ���ʹ�ֻ�����ʹ��һ���²ų�һ�ε硣
�ٸõ�ظ����ĵ缫��ӦʽΪ______________________________________��
�����Ըõ��Ϊ��Դ����ʯī���缫���200 mL�����������ӵ���Һ��
���� | Cu2�� | H�� | Cl�� | SO42�� |
c/(mol��L��1) | 0.5 | 2 | 2 | 0.5 |
���һ��ʱ��������ռ�����ͬ���(��ͬ������)������ʱ(������Һ����ı仯���缫������ܴ��ڵ��ܽ�����)�������ռ�������������Ϊ________��
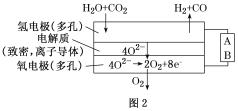
(4)���ˮ������CO2�����ϳ���(H2
���𰸡�2CH3OH(g)��O2(g)=2HCHO(g)��2H2O(g) ��H����316 kJ��mol��1�� ��״���ת���ʣ����������������Ϸ��ȣ�Ϊ�Ʊ���ȩ�ṩ���� ������� 1097 CH3OH��6e����8OH��===CO32����6H2O 3.2 g ���� H2O��2e��===H2����O2��
��������
(1). �� a CH3OH(g)===HCHO(g)��H2(g) ��H����84 kJ��mol��1��b 2H2(g)��O2(g)===2H2O(g) ��H����484 kJ��mol��1�����ݸ�˹���ɷ�����a��2+b���Ȼ�ѧ����ʽΪ��2CH3OH(g)��O2(g)===2HCHO(g)��2H2O(g) ��H��+84kJ/mol��2-484kJ/mol=��316 kJ��mol��1�� �ڸ��ݷ���ʽ2CH3OH(g)��O2(g)===2HCHO(g)��2H2O(g)��������Ӧ����ͨ���ʵ���������������״���ת���ʣ����������������Ϸ��ȣ�Ϊ�Ʊ���ȩ�ṩ������(2) �� ��Ӧ2CH3OH(g)��O2(g)===2HCHO(g)��2H2O(g)������a������b����ܼ�С��ӦΪ��������� �� CO(g)��2H2(g)==CH3OH(g)���ʱ�Ϊ��Ӧ���ܼ���֮��-�������ܼ���֮�ͣ���һ����̼��̼�����ļ���Ϊx�����ͼ���ṩ�Ļ�ѧ���ļ��ܣ���H��x+2��436kJ/mol-(3��413kJ/mol+358kJ/mol+463kJ/mol)=419kJ/mol-510kJ/mol=-91 kJ��mol��1������x=1097 kJ��mol��1��(3) ��ȼ�ϵ�أ���ص�һ���缫ͨ���������һ���缫ͨ��״����壬�����������������Һ������ԭ���ԭ���������缫��ӦΪ�״�ʧȥ��������̼������ӣ��缫��ӦΪ��CH3OH��6e����8OH��=CO32����6H2O ��������������ͭ���ӵõ��ӣ�����ͭ���ʣ�200mL0.5mol/L��ͭ���ӵõ������ʵ���Ϊ0.2mol��Ȼ���������ӵõ�������������������������0.4mol������ʧȥ���ӵķ�Ӧ����������0.2mol��ת�Ƶ�����0.4mol��Ȼ��������������ʧȥ���Ӳ����������������ռ�����ͬ���������ʱ������������������ʵ�����x���������ϲ�������ʱ��0.2+x��mol���ݵ����غ㣬�õ�0.4+4x=0.2+2(0.2+x)����x=0.1mol�����������ռ�������������Ϊ3.2 g�� (4)��ͼʾ��֪A��ˮ�Ͷ�����̼����������һ����̼��Ӧ������ԭ��Ӧ��Ϊ������Ӧ����AΪ��Դ�ĸ��������������ķ�ӦΪH2O��2e��=H2����O2����

 ���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�
���ٴ���������ѧϰ����ѧ�ں����ν�ϵ�д�