��Ŀ����
ijУ��ѧ�о���ѧϰС�飬��ѧϰ������ұ���Ժ��һ����̼��ԭ�����������ʵ��dz�����Ȥ�����Dz����й����Ϻ��֣�һ����̼���Ʊ������ü����Ũ���Ṳ�ȵ�60��80 �淢����ˮ��Ӧ��ȡ��
HCOOH CO����H2O
CO����H2O
��������¸�ͼ��������װ��һ����Ժ�����ʵ��װ��ͼ(ijЩװ�ÿ��ظ�ʹ��)��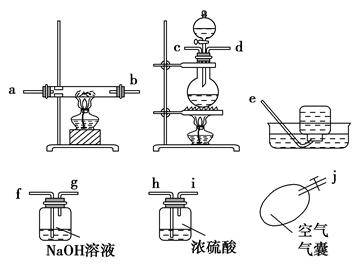
�ش��������⣺
(1)����ʵ��װ�õ�����˳����(дСд��ĸ) ��
(2)�ڷ�Ӧʱһ��Ҫ��ͨһ��һ����̼���壬Ȼ���ٵ�ȼ�����������ľƾ��ƣ�ԭ���� ��
| A����Ϊһ�㷴Ӧ�����ҽ��� |
| B���ų���ϵ�ڵĿ�����ʹ��Ӧ���̸���ȫ |
| C��������Ũ���ᷴӦ���Բ���������CO |
| D���˷�Ӧ����ʱ�䳤�����ڲ���CO |
(4)�ڹ۲쵽Ӳ�ʲ������е������� ɫ��ȫ��Ϊ ɫʱֹͣ���ȣ�Ȼ����� ��ԭ���Ƿ�ֹ����������
(5)Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ ��
(6)��ʵ���һ���ŵ��ǰ�ʵ������е�β��������ˮ���ռ��������ռ�������ǰ���м�ƿ���ֱ��ռ����Ⱥ�˳���ţ���ȼ����ƿ�е����壬�м��ŵļ���ƿ������ ��������Ⱥ����ļ���ƿ������ ��ԭ���� ��
(1)j��c��d��f��g��h��i��a��b��f��g��e��(2)B
(3)��ȥCO�е�����(����������HCOOH��CO2��SO2)
(4)���ء��ڡ�ͨһ����̼��װ����ȴ������
(5)Fe2O3��3CO 2Fe��3CO2
2Fe��3CO2
(6)����ȼ�գ���������ɫ���桡��ȼ�б�����(���л������)���ռ���������п���
����

���з�Ӧ����ʱ��������ػ�ɫ�̵��ǣ� ��
| A������������ȼ�� | B��ͭ��������ȼ�� |
| C��������������ȼ�� | D������������ȼ�� |
ʵ������ͭ�Ʊ�CuSO4��Һ�ж��ַ�����ijʵ��С��������������ַ�����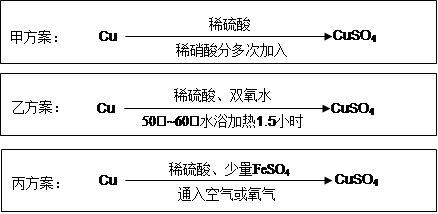
��ش��й����⣺
��1��������
��д���÷�Ӧ�����ӷ���ʽ ��
��Ϊ�˽�Լԭ�ϣ��������������ʵ���֮�����Ϊ��n(H2SO4)��n(HNO3)= ��
��2���ҷ�������6.4gͭ˿�ŵ�90mL 1.5mol��L-1��ϡ�����У�������50�档����40mL 10%��H2O2����Ӧ0.5Сʱ�����µ�60�棬������Ӧ1Сʱ��һϵ�в�������CuSO4��5H2O 20.0g����֪�й�Ħ���� ����M(Cu)=64g/mol�� M(CuSO4��5H2O) =250g/mol����
�ٷ�Ӧʱ�¶ȿ�����50��~60�棬���˹��ߵ�ԭ���� ��
�ڱ�ʵ��CuSO4��5H2O�IJ���Ϊ ��
��3����������������������ֱ��ͨ�뵽ͭ����ϡ����Ļ�����У������ڳ����¼�������Ӧ����ӦҺ�м�����FeSO4����������Ӧ����������ͭ����Ӧ��ȫ������A����pH��4 ��Ȼ����ˡ�Ũ�����ᾧ��
������A��ѡ�����µ� ������ţ���
| A��CaO | B��NaOH | C��CuCO3 | D��Cu2(OH)2CO3 E��Fe2(SO4)3 |
��4���Աȼס��ҡ�������ʵ�鷽�������������ŵ��У�д��������
�� ��
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
(1)���������һ�������ȼҵ��Ʒ������������������ķ������������£�
(��)����SO2�ķ���ͨ���ⱥ��ʳ��ˮ�����õ�����Һ�У���NaHSO3��Һ��
(��)����ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ
(��)���������NaHSO3��Һ�У���Ӧ���õ���SO2������գ����ɵ�NaClѭ�����á�
��д������(��)��Ӧ�Ļ�ѧ����ʽ�� ��
��д������(��)�е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ�� ��
��д������(��)��Ӧ�����ӷ���ʽ�� ��
(2)����ѧ���������Fe2����Fe3�������ӵĴ����ã������½�SO2������SO42-��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42-��ת���ʡ�
�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ������ ��(��д���)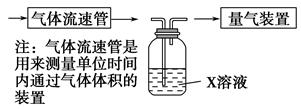
| A����ĵ�����Һ | B�����Ը��������Һ |
| C������������Һ | D���Ȼ�����Һ |
 ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� �� �������������ѧ�γ���������ǿ��,������������ͭ�ķ�Ӧ����ش���������:
(1)��100 mL 18 mol��L-1��Ũ�����м��������ͭƬ,����ʹ֮��ַ�Ӧ,�����������ڱ�״���µ������������������(��д����);
| A��7.32 L | B��6.72 L | C��20.16 L | D��30.24 L |







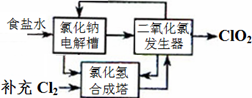
 2ClO2��+2Na2SO4��H2O
2ClO2��+2Na2SO4��H2O H++OH-�� ________________�������ӷ���ʽ��ʾ��.
H++OH-�� ________________�������ӷ���ʽ��ʾ��.