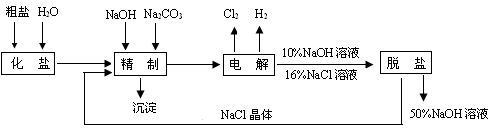
��Ŀ����
��������������Ⱦ��Ϊ���أ����������������ü�����⡣
(1)���������һ�������ȼҵ��Ʒ������������������ķ������������£�
(��)����SO2�ķ���ͨ���ⱥ��ʳ��ˮ�����õ�����Һ�У���NaHSO3��Һ��
(��)����ⱥ��ʳ��ˮ�������巴Ӧ���Ƶ����ᡣ
(��)���������NaHSO3��Һ�У���Ӧ���õ���SO2������գ����ɵ�NaClѭ�����á�
��д������(��)��Ӧ�Ļ�ѧ����ʽ�� ��
��д������(��)�е�ⱥ��ʳ��ˮ�Ļ�ѧ����ʽ�� ��
��д������(��)��Ӧ�����ӷ���ʽ�� ��
(2)����ѧ���������Fe2����Fe3�������ӵĴ����ã������½�SO2������SO42-��ʵ��SO2�Ļ������á�ij�о���ѧϰС��ݴ���������·�������ʵ���������²ⶨת������SO2������SO42-��ת���ʡ�
�ٸ�С�������ͼװ����ʵ���Ҳⶨģ��������SO2�����������X��Һ������ ��(��д���)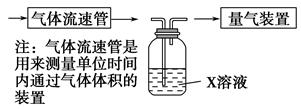
| A����ĵ�����Һ | B�����Ը��������Һ |
| C������������Һ | D���Ȼ�����Һ |
 ��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
��ת���ʣ���֪�������٣�����ⶨ�������� �� ��
(1)��SO2��NaOH=NaHSO3
��2NaCl��2H2O 2NaOH��H2����Cl2��
2NaOH��H2����Cl2��
��HSO3-��H��=SO2����H2O
(2)��A��B����ʵ��ʱ�䡡���������ữ��BaCl2��Һ�����ɳ���������
����

������һ����Ҫ�Ĺ�ҵԭ�ϡ���ҵ�����÷�Ӧ��3Cl2+8NH3=N2+6NH4Cl��������ܵ��Ƿ�©��������˵����ȷ����
| A�����ܵ�©�������ͻ�������� | B���÷�Ӧ�����˰����Ļ�ԭ�� |
| C���÷�Ӧ���ڸ��ֽⷴӦ | D������6molNH4Cl��18mol����ת�� |
������Ҫ�İ뵼����ϣ��������ִ����ӹ�ҵ�Ļ������ش��������⣺
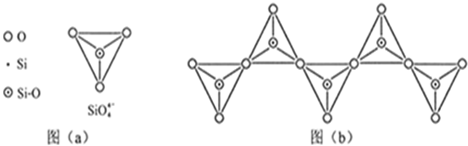
��1����̬Siԭ���У�����ռ�ݵ�����ܲ���� �����ܲ���е�ԭ�ӹ����Ϊ ��������Ϊ ��
��2������Ҫ�Թ����Ρ� �Ȼ��������ʽ�����ڵؿ��С�
��3�����ʹ��������ʯ�ṹ���Ƶľ��壬����ԭ����ԭ��֮���� ���ϣ��侧���й���8��ԭ�ӣ�����������λ�ù��� ��ԭ�ӡ�
��4�����ʹ��ͨ������(SiH4)�ֽⷴӦ���Ʊ�����ҵ�ϲ���Mg2Si��NH4CI��Һ�������з�Ӧ�Ƶ�SiH4���÷�Ӧ�Ļ�ѧ����ʽΪ ��
��5��̼����йػ�ѧ������������ʾ����Ҫ�����ͽ��������й���ʵ��
| ��ѧ�� | C-C | C-H | C-O | Si-Si | Si-H | Si-O |
| ���ܣ�KJ/mol�� | 356 | 413 | 336 | 226 | 318 | 452 |
��SiH4���ȶ���С��CH4���������������ԭ���� ��
��6���ڹ������У�SiO44�������壨����ͼa��ͨ�����ö��������ӿ��γɵ�״����״����״���Ǽ���״�Ĵ���ṹ��ʽ��ͼbΪһ�����������ṹ�Ķ�����������Siԭ�ӵ��ӻ���ʽΪ ��Si��O��ԭ����֮��Ϊ ��ѧʽΪ ��
ijУ��ѧ�о���ѧϰС�飬��ѧϰ������ұ���Ժ��һ����̼��ԭ�����������ʵ��dz�����Ȥ�����Dz����й����Ϻ��֣�һ����̼���Ʊ������ü����Ũ���Ṳ�ȵ�60��80 �淢����ˮ��Ӧ��ȡ��
HCOOH CO����H2O
CO����H2O
��������¸�ͼ��������װ��һ����Ժ�����ʵ��װ��ͼ(ijЩװ�ÿ��ظ�ʹ��)��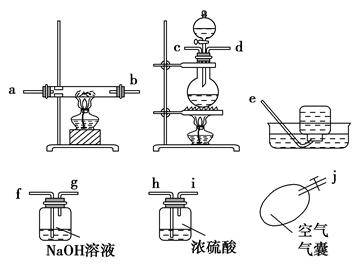
�ش��������⣺
(1)����ʵ��װ�õ�����˳����(дСд��ĸ) ��
(2)�ڷ�Ӧʱһ��Ҫ��ͨһ��һ����̼���壬Ȼ���ٵ�ȼ�����������ľƾ��ƣ�ԭ���� ��
| A����Ϊһ�㷴Ӧ�����ҽ��� |
| B���ų���ϵ�ڵĿ�����ʹ��Ӧ���̸���ȫ |
| C��������Ũ���ᷴӦ���Բ���������CO |
| D���˷�Ӧ����ʱ�䳤�����ڲ���CO |
(4)�ڹ۲쵽Ӳ�ʲ������е������� ɫ��ȫ��Ϊ ɫʱֹͣ���ȣ�Ȼ����� ��ԭ���Ƿ�ֹ����������
(5)Ӳ�ʲ������з�����Ӧ�Ļ�ѧ����ʽΪ ��
(6)��ʵ���һ���ŵ��ǰ�ʵ������е�β��������ˮ���ռ��������ռ�������ǰ���м�ƿ���ֱ��ռ����Ⱥ�˳���ţ���ȼ����ƿ�е����壬�м��ŵļ���ƿ������ ��������Ⱥ����ļ���ƿ������ ��ԭ���� ��
ij��ѧС�����Na2SO3������ʵ��̽����
�ڰ�ɫ��ΰ��a��b��c���������е���Na2SO3��Һ���ٷֱ�μ���ͼ��ʾ���Լ���
ʵ���������±���
| ��� | ʵ������ |
| a | ��ˮ��ɫ |
| b | ��������ɫ���� |
| c | �����̪��Һ��죬�ټ���BaCl2��Һ����������Һ�ɫ��ȥ |
����ʵ��������з�����
(1)a��ʵ������֤��Na2SO3����________�ԡ�
(2)b�з�����Ӧ�����ӷ���ʽ��_____________________________________________
(3)Ӧ�û�ѧƽ��ԭ������c������(�û�ѧ���P�����ֱ���)__________________________________________________________��