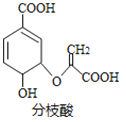
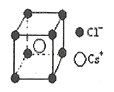��Ŀ����
����Ŀ����֪Ԫ��N��S��![]() ���γɶ������ʣ��ڹ�ҵ���������Ź㷺��Ӧ�á���ش��������⣺
���γɶ������ʣ��ڹ�ҵ���������Ź㷺��Ӧ�á���ش��������⣺
��1��Se��S��ͬ��Ԫ�أ���д����̬Seԭ�ӵĵ����Ų�ʽ��__��N��S�Dz�ͬ��Ԫ�أ������NH3��ˮ�е��ܽ�ȱ�H2S���ԭ��__��
��2����һ����1~9��Ԫ���еIJ���Ԫ����ɣ�����SCl2��Ϊ�ȵ�����Ĺ��ۻ�������ķ���ʽΪ__�������ȵ�����ԭ�����Է�����SCN-�������������ĸ�����Ϊ__��
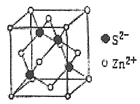
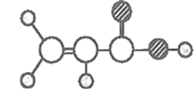
��3����֪![]() �ĽṹΪ
�ĽṹΪ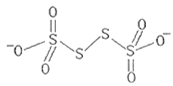 ����Sԭ�ӵ��ӻ���ʽ��__��
����Sԭ�ӵ��ӻ���ʽ��__��
��4��N��P�ɷֱ��γɶ��������η��ӣ���֪NH3�ļ��Ǵ���PH3��ԭ����__��
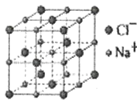
��5�����Ӿ����������Ӻ������ӵİ뾶�Ȳ�ͬ���γɲ�ͬ�ľ����ṹ�����±���
�뾶�� | 0.225��0.414 | 0.414��0.732 | 0.732��1 |
���ͻ�ѧʽ | ����ZnS | NaCl | CsCl |
���� |
|
|
|
��֪ij���Ӿ���RA�����������Ӱ뾶�ֱ�Ϊ184pm��74pm��Ħ������ΪMg/mol������������λ��Ϊ__��������ܶ�Ϊ__g/cm3���г�����ʽ�����軯����NAΪ�����ӵ�������ֵ����
���𰸡�[Ar]3d104s24p4��1s22s22p63s23p63d104s24p4 NH3������H2O����֮������γ��������H2S���Ӳ�����H2O����֮���γ���� OF2 1��1 sp3�ӻ� ���ڵ縺��N>P>H���ҵ�ԭ�Ӱ뾶С����ԭ�Ӱ뾶��NH3�����гɼ����ӶԱ˴��������������������Լ���NH3>PH3 4 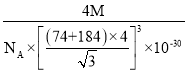
��������
��1��Ԫ��Se���ڵ�������VIA�壬ԭ������Ϊ34��NH3������H2O���Ӽ��γ������H2S������H2O�γ������
��2���ȵ�������ָԭ����Ŀ��ȣ��۵�����Ŀ��ȵ����ӣ�����ԭ���滻��д��ͬ����Ԫ��ԭ�Ӽ۵�����Ŀ��ȣ�
��3��![]() �Ľṹ�����ߵ�Sԭ�Ӿ��γ�4���Ҽ����¶Ե��ӣ��м������Sԭ�Ӿ��γ������������Ҿ������Թ¶Ե��ӣ�
�Ľṹ�����ߵ�Sԭ�Ӿ��γ�4���Ҽ����¶Ե��ӣ��м������Sԭ�Ӿ��γ������������Ҿ������Թ¶Ե��ӣ�
��4���縺��N��P����Nԭ�Ӱ뾶С��Pԭ�Ӱ뾶��NH3�гɼ����ӶԱ˴������������������
��5������74��184��0.402�����Ӿ���RA�ľ���Ϊ����ZnS�ͣ������������ӵ���λ��Ϊ4���������������Ӹ�����Ϊ1��1.1����������4����RA�������Ħ���������㾧���������������������Ӱ뾶֮��Ϊ��Խ��ߵ�![]() ������Խ���Ϊ�����߳���
������Խ���Ϊ�����߳���![]() ��������ܶȹ�ʽ����
��������ܶȹ�ʽ����
��1��Ԫ��Se���ڵ�������VIA�壬ԭ������Ϊ34����̬�����Ų�Ϊ1s22s22p63s23p63d104s24p4��N�ĵ縺�Ժܴ�ʹ��NH3��N-H�����Էdz�ǿ��Nԭ�Ӵ������Եĸ���ɣ�H2Oǿ����O-H�е�H������Ϊ��¶�����ӣ�NH3������H2O���Ӽ��γ���O-H��N�����ʹNH3��ˮ�кܴ���ܽ�ȡ�H2S��S��������ɲ�����ʹH2S��H2O�γ����������H2S��ˮ���ܽ�Ƚ�С���ʴ�Ϊ��[Ar]3d104s24p4��1s22s22p63s23p63d104s24p4��NH3������H2O����֮������γ��������H2S���Ӳ�����H2O����֮���γ������
��2���ȵ�������ָԭ����Ŀ��ȣ��۵�����Ŀ��ȵ����ӣ���1��9��Ԫ���еIJ���Ԫ����ɵ������У���SCl2��Ϊ�ȵ�����Ĺ��ۻ����ֻ��OF2��SCN-��CO2��Ϊ�ȵ����壬CO2�ķ��ӽṹΪO=C=O�����ЦҼ��ͦм��ĸ�����Ϊ2��2=1��1������SCN-�ЦҼ��ͦм��ĸ�����ΪҲ1��1���ʴ�Ϊ��OF2��1��1��
��3��![]() �Ľṹ�����ߵ�Sԭ�Ӿ��γ�4���������¶Ե��ӣ����Ծ�Ϊsp3�ӻ����м������Sԭ�Ӿ��γ������������Ҿ������Թ¶Ե��ӣ����Ծ�Ϊsp3�ӻ����ʴ�Ϊ��sp3�ӻ���
�Ľṹ�����ߵ�Sԭ�Ӿ��γ�4���������¶Ե��ӣ����Ծ�Ϊsp3�ӻ����м������Sԭ�Ӿ��γ������������Ҿ������Թ¶Ե��ӣ����Ծ�Ϊsp3�ӻ����ʴ�Ϊ��sp3�ӻ���
��4��NH3��PH3���������νṹ������һ�Թ¶Ե��ӵij���Ӱ�죬���ڵ縺��N��P��H����Nԭ�Ӱ뾶С��Pԭ�Ӱ뾶��NH3�гɼ����ӶԱ˴���������������������Լ���NH3��PH3���ʴ�Ϊ�����ڵ縺��N>P>H���ҵ�ԭ�Ӱ뾶С����ԭ�Ӱ뾶��NH3�����гɼ����ӶԱ˴��������������������Լ���NH3>PH3��
��5������74��184��0.402�����Ӿ���RA�ľ���Ϊ����ZnS�ͣ������п��Կ��������ӵ���λ��Ϊ4�������������Ӹ�����Ϊ1��1�����������ӵ���λ��Ϊ4��1����������4����RA������������������=![]() ���������Ӱ뾶֮��Ϊ��Խ��ߵ�
���������Ӱ뾶֮��Ϊ��Խ��ߵ�![]() ������Խ���Ϊ�����߳���
������Խ���Ϊ�����߳���![]() ���������ܶ�=
���������ܶ�=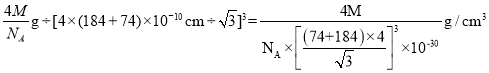 ���ʴ�Ϊ��4��
���ʴ�Ϊ��4��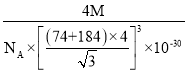 ��
��

 ��У����ϵ�д�
��У����ϵ�д�����Ŀ���±��Ǽס��ҡ������������л�����й���Ϣ��
�� | ����ʹ������Ȼ�̼��Һ��ɫ�� ������ˮ��һ�������·�Ӧ���ɱ��� �۱���ģ��Ϊ |
�� | ����C��H����Ԫ����ɣ� �ڱ���ģ��Ϊ |
�� | ����C��H��O����Ԫ����ɣ� ������Na��Ӧ����������NaOH��Һ��Ӧ�� �����붡��Ӧ������Է�������Ϊ100���� |
�� | ����C��H��O����Ԫ����ɣ� �����ģ��Ϊ |
�ش��������⣺
��1������������Ȼ�̼��Һ��Ӧ��������Ľṹ��ʽ��__��
��2���Ҿ��е�������__������ţ���
A.��ɫ��ζҺ�壬�ж�
B.������ˮ���ܶȱ�ˮ�Ĵ�
C.����ʹ����KMnO4��Һ��ɫ
D.�κ������²���������Ӧ
��3�����Ĺ����ŵ����ƣ�__��д������Na��Ӧ�Ļ�ѧ����ʽ��__��
��4���������������ӳɷ�Ӧ�����������죬�����ڽṹ�����Ƶ��л�����һ���ࣨ����ͬϵ�����������Ǿ�����ͨʽCnH2n+2����n��__ʱ�������л������ͬ���칹����
��5�����붡��Ӧ��������Է�������Ϊ100�������÷�Ӧ�ķ�Ӧ����Ϊ__���仯ѧ����ʽΪ___��
����Ŀ������ʵ�鷽�����ܴﵽԤ��Ŀ�ĵ���
ʵ�鷽�� | ʵ��Ŀ�� | |
A | ����֧�Թ��зֱ���� | ̽�� |
B | ȡ | ����8%�� |
C | �� | �Ƚ� |
D | ��ͬ��ͬŨ�� | �Ƚ������Ԫ�طǽ�����ǿ�� |
A.AB.BC.CD.D