��Ŀ����
1808�꣬Ӣ����ѧ�Ҵ�ά�üػ�ԭ����þ�������Ƶ�������þ��þ�Ǻ��չ�ҵ����Ҫ���ϣ�þ�Ͻ���������ɻ�����������������ȣ�һ�ܳ����ٷɻ�Լ��5%��þ�Ͻ���һö����һ������100��200����þ�Ͻ�þ��Ϊһ��ǿ��ԭ�����������ѡ�ﯡ�����˵ȵ������У�þ��ȼ�յ�������������ȱ�ٵ�����þ���ǽ����̻������ԭ�ϡ�þ����Ͻ���һ����;�ܹ㷺�Ľ������ϣ�Ŀǰ������60%��þ�ǴӺ�ˮ����ȡ�ġ��Ӻ�ˮ����Ҫ��NaCl��MgSO4������ȡ����þ����Ҫ�������£�
�ش��������⣺
(1)Mg�����ڱ��е�λ��______________________���Լ�A����ѡ��_____________________���Լ�C��ѡ��_______________________��
(2)����ٵ����ӷ���ʽ_________________________________________��
(3)�õ���ʽ��ʾ�Ȼ�þ���γɹ���_______________________________��
(4)��ˮMgCl2������״̬�£�ͨ�������Mg��Cl2,�÷�Ӧ�Ļ�ѧ����ʽΪ��_____________________________________________________��
(5)��ά��þ�Ļ�ѧ����ʽΪ_____________________________________��
(6)��ƽ���з�Ӧ�ķ���ʽ��
�� 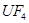 +
+ 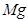 ����
����  +
+ 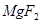
�� 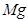 +
+ 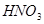 ����
���� 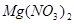 +
+ 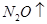 +
+ 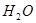
��16�֣�ÿ��2�֣�(1) �������ڵڢ�A�壬 Ca(OH)2��Һ ����
(2) 2OH- + Mg2+�� Mg(OH)2��
(3) 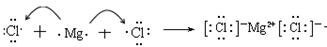
(4) MgCl2(����) Mg + Cl2�� (5)2K + MgO
Mg + Cl2�� (5)2K + MgO  K2O + Mg
K2O + Mg
(6) 1��2��1��2 4��10��4��1��5
���������������1��þ��ԭ��������12��λ��Ԫ�����ڱ��ĵ������ڵڢ�A�塣��ˮ�е�þ����ת��������Ӧ�ü���ʯ�����������ƣ���þ����ת��Ϊ������þ������������þת��Ϊ�Ȼ�þ����Ҫ��������þ�ܽ��������С�
��2���������Ϸ�����֪������ٵ����ӷ���ʽ��2OH- + Mg2+��Mg(OH)2����
��3���Ȼ�þ�Ǻ������Ӽ������ӻ�������γɹ��̿ɱ�ʾΪ ��
��
��4����ͨ��������£�������ڵ��Ȼ�þ���������ͽ���þ����Ӧ�ķ���ʽ��MgCl2(����) Mg + Cl2����
Mg + Cl2����
��5���ڸ����£�����K������þ�����û���Ӧ�����ɽ���þ����Ӧ�Ļ�ѧ����ʽ��2K + MgO  K2O + Mg��
K2O + Mg��
��6�����ڷ�Ӧ��Mg�Ļ��ϼ۴�0�����ߵ���2�ۣ�ʧȥ2�����ӡ�UԪ�صĻ��ϼ۴ӣ�4�۽��͵�0�ۣ��õ�4�����ӣ����Ը��ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ���ʽ�� 1 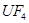 + 2
+ 2 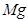 �� 1
�� 1  + 2
+ 2 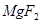 ��
��
�ڸ��ݷ���ʽ��֪��Mg�Ļ��ϼ۴�0�����ߵ���2�ۣ�ʧȥ2�����ӡ������е�Ԫ�صĻ��ϼ۴ӣ�5�۽��͵���1�ۣ��õ�4�����ӣ�����ݵ��ӵĵ�ʧ�غ��֪����ƽ��ķ���ʽ�� 4 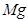 + 10
+ 10 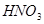 �� 4
�� 4 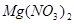 + 1
+ 1 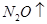 + 5
+ 5 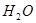 ��
��
���㣺���麣ˮ�н���þ����ȡ������þ�����ڱ��е�λ�á��Ȼ�þ���γɡ�������ԭ��Ӧ����ʽ����ƽ
�����������Ǹ߿��еij������ͣ������е��Ѷȵ����⡣�����ۺ���ǿ�����ض�ѧ�������������ͽ��ⷽ����ָ����ѵ����ּ�ڿ���ѧ��������û���֪ʶ���ʵ�����������������������ѧ����Ӧ�������������������������Ժ�ˮ���ۺ�Ӧ��Ϊ���壬�����ڵ���ѧ����ѧϰ��Ȥ������ѧ����ѧϰ��֪����

����15N���Բⶨ�������ζԵ���ˮ�ʵ���Ⱦ�����
��1������˵����ȷ����___��
| A��14N��15NΪͬ�ֺ��� |
| B��14N�ĵ�һ������С��14C |
| C��15NH3��14NH3�����γɷ��Ӽ���� |
| D��CH315NH2��CH3CH214NH2��Ϊͬϵ�� |
��3�� ��ҵ�ϵ�ⷨ�����������ε�ģ�����ͼ��

��֪��������������ѧ��Ӧ��
2NO2-+8H++6Fe2+==N2�� +6Fe3++4HzO
�������缫��Ӧ����ʽ��______(�����Ǻ�����Ӧ����
����������ҺŨ�ȹ������������������ݳ���������Ϊ______(�ѧʽ��;���������c( H+ )Խ��H+������Խ______;
�������ʱ��·����0.6 mol����ת��,��NaNO2��ʣ��,�����������Na2SO4______mol��
��13�֣��ۻ�ѧ�������ʽṹ�����ʣ���Cu��N��B��Ԫ����ɵ����Ͳ������Ź㷺��;��
��1����̬ Cu+ �ĺ�������Ų�ʽΪ ���ڸ�����CuO �ֽܷ�����Cu2O���Դ�ԭ�ӽṹ�ǶȽ�����ԭ�� ��
��2��������������һ�����͵ij�Ӳ����ĥ�����µĽṹ���ϣ��������� �����塣
��3��������A (H3BNH3) ��һ��DZ�ڵĴ�����ϣ���������Ԫ��״������ (HB=NH)3ͨ��3CH4 + 2 (HB=NH)3 + 6H2O ��3CO2 + 6H3BNH3 �Ƶá�
����������ѧ����ʽ�йص���������ȷ���� �������ţ�
| A����Ӧǰ��̼ԭ�ӵĹ���ӻ����Ͳ��� |
| B��CH4��H2O��CO2���ӿռ乹�ͷֱ��ǣ����������Ρ�V�Ρ�ֱ���� |
| C����һ�����ܣ�N��O��C��B |
| D��������A�д�����λ�� |
��4�����������У�����������״����״�ȶ��ֽṹ��ʽ��ͼ��a����һ����״�ṹ�Ķ�������������������ӷ���Ϊ���� ��ͼ��b������ɰ�����������ӵĻ�״�ṹ��������ԭ�Ӳ�ȡ���ӻ�����Ϊ�� ������

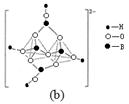
ijѧ������Ԫ��������ԭ�ӽṹ�Ĺ�ϵ��ʵ��ʱ�������һ��ʵ�鷽��������¼���йص�ʵ�������������ѧ�����������ʵ�鱨�档
��1��ʵ��Ŀ�ġ�
̽��ͬһ����Ԫ�����ʵĵݱ���ɡ�
��2��ʵ����Ʒ��
�������Թܡ���ͷ�ιܡ�
ҩƷ��������ˮ��������ˮ���廯����Һ���⻯����Һ�����Ȼ�̼��
��3��ʵ�����ݣ����±���������д������ݣ���
| ��� | ʵ�鷽�� | ʵ������ |
| �� | ��ʢ�������廯����Һ���Թ��еμ�����������ˮ�����ټ����������Ȼ�̼������ | Һ���Ϊ���㣬�²��________ɫ |
| �� | ��ʢ�������⻯����Һ���Թ��еμ�����������ˮ�����ټ����������Ȼ�̼������ | Һ���Ϊ���㣬�²��________ɫ |
��4��ʵ����ۣ�__________________________________________________________��
��5����������ۡ�
����������ʵ���з�����Ӧ�����ӷ���ʽ�ֱ�Ϊ________��________��
�����ڷ����ʹ��ڻ��ã����Ժ�����Ƴ�һ����ʵ������֤�������Ե�ǿ�������о�������ʵ˵�����ķǽ����Ա��ȵ�ǿ��________��________��


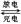 Cd(OH)2�� 2Ni(OH)2
Cd(OH)2�� 2Ni(OH)2
 ����м�����Ŀ֮��Ϊ_________��
����м�����Ŀ֮��Ϊ_________��