��Ŀ����
����Ŀ���л�������P�Ǻϳɿ�����ҩ����м��壬��ϳ�·�����£�

��֪�� ![]()
(1)A����NaHCO3��Һ��Ӧ��д��A�Ĺ���������_____��
(2)A��B�ķ�Ӧ����_____��
(3)C��D�Ļ�ѧ����ʽ_____��
(4)����F�й����ŵ��Լ�������_____��
(5)G��H������Լ�a��______��
(6)D��E�Ļ�ѧ����ʽ______��
(7)��֪��
K�����ಽ��Ӧ���յõ�����P��

��L�Ľṹ��ʽ___________��
��Q��M�Ļ�ѧ����ʽ___________��
���𰸡��Ȼ� ȡ����Ӧ HOOC-CH2-COOH+2C2H5OH![]() C2H5OOC-CH2-COOC2H5+2H2O FeCl3��Һ����Һ����ɫ(��Ũ��ˮ�����ɰ�ɫ����) ŨH2SO4 ��ŨHNO3 C2H5OOC-CH2-COOC2H5+CH(OC2H5)3
C2H5OOC-CH2-COOC2H5+2H2O FeCl3��Һ����Һ����ɫ(��Ũ��ˮ�����ɰ�ɫ����) ŨH2SO4 ��ŨHNO3 C2H5OOC-CH2-COOC2H5+CH(OC2H5)3![]() (C2H5OOC)2C=CHOC2H5+2C2H5OH
(C2H5OOC)2C=CHOC2H5+2C2H5OH 

��������
A����ʽ��C2H4O2��������NaHCO3��Һ��Ӧ����A��CH3COOH��A��Cl2�ڴ�������ʱ�������ϵ�ȡ����Ӧ����B��ClCH3COOH��B��NaCN��H+����ʱ������Ӧ����C��HOOC-CH2-COOH��C��C2H5OH��Ũ�������ʱ������������Ӧ����D��C2H5OOC-CH2COOC2H5��D��CH(OC2H5)3������Ӧ����E��(C2H5OOC)2C=CHOC2H5��CH3CH2OH��F����ʽ��C6H6O�����G�ķ��ӽṹ�����������CH3I����֪F�DZ��ӣ�G��![]() ��G��Ũ���ᡢŨ�����ϼ��ȷ���ȡ����Ӧ������H��
��G��Ũ���ᡢŨ�����ϼ��ȷ���ȡ����Ӧ������H��![]() ��H����ԭ����J��
��H����ԭ����J��![]() ��J��E��Ӧ����K��
��J��E��Ӧ����K��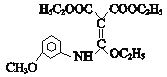 ��K����һϵ�б仯����P��
��K����һϵ�б仯����P��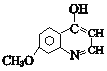 ���ݴ˷������
���ݴ˷������
(1) A��CH3COOH������NaHCO3��Һ��Ӧ����A�Ĺ������������Ȼ���
(2)A��CH3COOH��A��CH3I����ȡ����Ӧ����B��ClCH2-COOH����A��B�ķ�Ӧ������ȡ����Ӧ��
(3)C�DZ�����HOOC-CH2-COOH��C���Ҵ���Ũ�������ʱ���ڼ��������·���������Ӧ����D���������������C2H5OOC-CH2-COOC2H5����C��D�Ļ�ѧ����ʽΪ��HOOC-CH2-COOH+2C2H5OH![]() C2H5OOC-CH2-COOC2H5+2H2O��
C2H5OOC-CH2-COOC2H5+2H2O��
(4)F�DZ��ӣ��������Ƿ��ǻ�������F�й����ŵ��Լ�������FeCl3��Һ������Һ�м���FeCl3��Һ����Һ��Ϊ��ɫ��Ҳ��������Һ�м���Ũ��ˮ����۲쵽�����������ɰ�ɫ������
(5)G�DZ�����![]() �������м���Ũ���ᡢŨ���ᣬ�����ȣ��ᷢ����OCH3ȡ������λ�ϵ�ȡ����Ӧ����NO2ȡ����OCH3��λ�ϵ�Hԭ�ӣ�����Hԭ�ӣ����G��H������Լ�a��ŨH2SO4 ��ŨHNO3��
�������м���Ũ���ᡢŨ���ᣬ�����ȣ��ᷢ����OCH3ȡ������λ�ϵ�ȡ����Ӧ����NO2ȡ����OCH3��λ�ϵ�Hԭ�ӣ�����Hԭ�ӣ����G��H������Լ�a��ŨH2SO4 ��ŨHNO3��
(6)D��C2H5OOC-CH2-COOC2H5��D��CH(OC2H5)3������Ӧ����E��(H5C2OOC)2C=CHOC2H5��CH3CH2OH����D��E�Ļ�ѧ����ʽ��C2H5OOC-CH2-COOC2H5+CH(OC2H5)3![]() (H5C2OOC)2C=CHOC2H5+2C2H5OH��
(H5C2OOC)2C=CHOC2H5+2C2H5OH��
(7)��K�ṹ��ʽΪ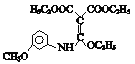 ��������Ŀ��Ϣ��֪K��һ�������·��������ϵ�ȡ����Ӧ����L��
��������Ŀ��Ϣ��֪K��һ�������·��������ϵ�ȡ����Ӧ����L�� ��L��POCl3������Ӧ����Q��
��L��POCl3������Ӧ����Q��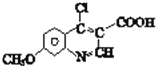 ��
��
��Q��NaOHˮ��Һ�ڼ���ʱ������Ӧ����M��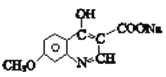 ��Q��M�Ļ�ѧ����ʽΪ��
��Q��M�Ļ�ѧ����ʽΪ�� ��
��

����Ŀ������ƽ�ⳣ���Ǻ���������ʵ���̶ȵ�����������֪�������(25��)��
��ѧʽ | ����ƽ�ⳣ�� |
HCN | K=4.9��10-10 |
CH3COOH | K=1.8��10-5 |
H2CO3 | K1=4.4��10-7��K2=4.7��10-11 |
(1)25��ʱ���������ʵ���Ũ����ͬ��������Һ��pH�ɴ�С��˳��Ϊ_____(����ĸ����ͬ)��
a��NaCN��Һ b��Na2CO3��Һ c��CH3COONa��Һ d��NaHCO3��Һ
(2)25��ʱ����NaCN��Һ��ͨ������CO2���������Ļ�ѧ����ʽΪ____________________��
(3)�����ʵ�����Na2CO3��NaHCO3�����Һ�и�����Ũ���ɴ�С��˳��Ϊ____________________��
(4)���ڴ�����Һ�ʹ�������Һ������˵����ȷ����__________��
a��ϡ�ʹ�����Һ������ĵ���̶�����ϡ�ʹ�������Һ������Ƶ�ˮ��̶ȼ�С
b�������¶ȿ��Դٽ�������룬�������¶Ȼ����ƴ�����ˮ��
c������ʹ����ƵĻ��Һ�У�����ٽ������Ƶ�ˮ�⡢������Ҳ�ٽ�����ĵ���
d������ʹ����ƵĻ��Һ�У��������ƴ����Ƶ�ˮ�⡢������Ҳ���ƴ���ĵ���
(5)25������֪pH = 2�ĸߵ�����Һ��pH = 12��NaOH��Һ�������������û��Һ��������0.01 mol/L�ĵ�����������Һ��pH = 12��NaOH��Һ�������������û��Һ�����ԡ���ߵ�����____________(����ǿ��������������)����������___________(����ǿ��������������)���������Եĸߵ��ᱵ[Ba5(IO6)2]��ϡ�����ϣ�������Ӧ�����ӷ���ʽΪ��__________________________________________________��
(6)����ʯ������CO2���������ܵ����CaCO3�����ܶȻ�����Ksp=2.8��10-9������һ���ʵ���Ũ��Ϊ2��10-4mol/L������Һ��������������CaCl2��Һ��ϣ������ɳ�������CaCl2��Һ����СŨ��Ϊ______mol/L��
����Ŀ���̵Ļ������ڹ�ҵ��ҽ�Ƶ���������ҪӦ�á�ij��ȤС��ģ���Ʊ�KMnO4��̽����(II)���ܷ�����Ϊ����(VII)���Ρ�
I��KMnO4���Ʊ���
![]()
��Ӧԭ��
����һ��3MnO2+6KOH+KClO3![]() 3K2MnO4+KCl+3H2O
3K2MnO4+KCl+3H2O
�������3K2MnO4+2CO2 =2KMnO4+MnO2+2K2CO3
ʵ�����
����һ����һ��������MnO2��KOH��KClO3 �����ϼ��ȣ��õ�ī��ɫ�Ĺ��壬��ȴ���ˮ�ܽ�õ�����K2MnO4��Һ�����ձ�C�С�
�����������װ�ã���������Ժ�װҩƷ����Һ©����������C����Һ��ȫ��Ϊ�Ϻ�ɫʱ���رջ���ֹͣ��Ӧ�����롢�ᴿ��ȡKMnO4���塣
װ��ͼ���£�

(1)���װ��A�����ԣ��رշ�Һ©����������B�м�������ˮ��Һ�泬�������ܿڣ�����ë����סԲ����ƿ����__________����˵��װ�����������á�
(2)B���Լ�ѡ�������________��
(3)��Ӧ������δ�ܼ�ʱ����KMnO4���壬����C���Ϻ�ɫ��Һ��dz����С��ͬѧ��Ϊ�Ǽ���K2MnO4��Һ�е�Cl-�����ɵ�MnO4- ��ԭ��������ɫ��dz��ָ������Cl-���ж�����_____________��
II����С�����̽��Mn2+�ܷ�����ΪMnO4-������������ʵ�飺
װ��ͼ | �Լ�X | ʵ������ |
| �� 0.5mL 0.1mol/LNaOH��Һ | ����dz��ɫ������һ��ʱ����Ϊ�غ�ɫ |
��0.5mL 0.1mol/LNaOH ��15%H2O2 ���Һ | ���������غ�ɫ���� | |
�� 0.5mL 0.1mol/LHNO3��Һ | ���������� | |
�� 0.5mL 0.1 mol/LHNO3��Һ������PbO2 | �μ�HNO3������������PbO2������Ϊ�Ϻ�ɫ���Ժ��Ϻ�ɫ��ʧ�������غ�ɫ���� |
��֪��i.MnO2Ϊ�غ�ɫ���壬������ˮ��
iiKMnO4�����Ի����»����ֽ����MnO2��
(4)ʵ����������غ�ɫ�������ܵ�ԭ��_____��
(5)ʵ�����Ѹ�������غ�ɫ���������ӷ���ʽ_____��
(6)�Ա�ʵ��ۺ͢ܣ�ʵ��۵�����_____��
(7)��ͬѧ�²�ʵ������Ϻ�ɫ��ʧ��ԭ������������KMnO4���ȶ����ֽ������MnO2������Ϊ��������������________������Ϊ����Һ�е�Mn2+��MnO4- ��ԭ���������ʵ�鷽��֤�����Ʋ�������䷽��Ϊ_________��
̽������������������£�ijЩǿ���������Խ�Mn2+����ΪMnO4-��
����Ŀ��ʵ��С���Ʊ�����������(Na2S2O5)��̽�������ʡ�
���ϣ�����������Ϊ��ɫ���壻������ˮ��
(1)�Ʊ�Na2S2O5(�г�װ����)

�� AΪSO2����װ�ã�A�з�Ӧ����ʽ��_____________________________________��
�� B ��������ɫ���壬�ɲ�ȡ�ķ��뷽����___________________��
�� ��β������װ��C�������������������Լ���___________________
(2) ̽��Na2S2O5������
ʵ���� | ʵ����������� |
ʵ��� | ȡB��������ɫ�������Թ��У�������������ˮ������Һa�������Һ�����ԡ� |
ʵ��� | ȡ������Һa���Թ��У��μ���������������Һ���а�ɫ�������ɣ����˺����������Թ��У��μӹ������ᣬ������������ݣ���ɫ�����ܽ⡣ |
ʵ��III | ȡB��������ɫ�������Թ��У��μ�1mL 2 mol / L����KMnO4��Һ�����ҷ�Ӧ����Һ�Ϻ�ɫ�ܿ���ȥ �� |
ʵ��IV | ȡB��������ɫ�����ڴ��Թ��м��ȣ�������������ͨ��Ʒ����Һ�У���ɫ��ȥ������ɫ�����Һ���ȣ���ɫ�ָ��� |
�� ��ʵ����֪��Na2S2O5����ˮ����Һ�����Ե�ԭ��_______________________________________(�û�ѧ����ʽ��ʾ) ��
�� ʵ����а�ɫ�����μӹ������ᣬ�����ܽ⣬��ƽ��ԭ������ԭ��______________________��
�� ʵ��III�о��ⶨ��Һ�в���Mn2+���÷�Ӧ�����ӷ���ʽ��_________________________��
�� ʵ��IV �в���������Ϊ____________��ʵ�������ø������_________ ���ʽ��м��顣
�� ������ʵ��̽���У����ֳ�Na2S2O5���� _____________________���ʡ� ( ����˵������ )��




