��Ŀ����
����Ŀ��ij�������ֻ������Ե����̼�������缫���ϣ�������ZnSO4��Һ���л��߾�������̬����ʣ������ܷ�ӦΪMnO2+![]() Zn+(1+
Zn+(1+![]() )H2O+
)H2O+![]() ZnSO4
ZnSO4![]() MnOOH+
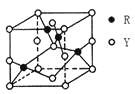
MnOOH+![]() ZnSO4[Zn(OH)2]3��xH2O.���ؽṹ��ͼ1��ʾ��ͼ2���л��߾���ĽṹƬ�Ρ�����˵���У�����ȷ���ǣ� ��
ZnSO4[Zn(OH)2]3��xH2O.���ؽṹ��ͼ1��ʾ��ͼ2���л��߾���ĽṹƬ�Ρ�����˵���У�����ȷ���ǣ� ��

A.̼���ܾ��е����ԣ��������缫����
B.�ŵ�ʱ��Zn2+����MnO2Ĥ
C.���ʱ����ص�������ӦΪMnOOH+e- =MnO2 + OH-
D.�л��߾���ĵ���![]() �к���̼̼˫��
�к���̼̼˫��
���𰸡�C
��������
A.�õ����̼�������缫���ϣ���֪̼���ܾ��е����ԣ�ѡ��A��ȷ��
B.�ŵ�ʱ��װ��Ϊԭ��أ���������������������Zn2+����MnO2Ĥ��ѡ��B��ȷ��
C.���ʱ��ص���������ʧ���ӵ�������Ӧ����缫��ӦʽΪ��MnOOH-e-+OH-=MnO2+H2O��ѡ��C����.
D.���ݸ߾���Ľṹ��Ԫ���ø߾���Ϊ�Ӿ۲���ϳ��л��߾���ĵ����ǣ�CH2=CH-CO-NH2������̼̼˫��,ѡ��D��ȷ��
��ѡC��

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�����Ŀ��ijͬѧ����֪Ũ�ȵ��������ⶨij����������Ʒ�Ĵ��ȣ���־�����뷴Ӧ�����Ը���ʵ��ش��������⣺
��1��ȷ����5.0 g���������������ʵ���Ʒ�����1L������Һ������ʱ����Ʒ�ɷ���________(����ĸ)������
A.С�ձ��С������� B.�ྻֽƬ�ϡ����� C.������
��2���ζ�ʱ����0.100 0 mol��L��1���������ζ�������Һ����ѡ��________��ָʾ����
A.���� B.ʯ�� C.��̪
��3���ζ�����������������Һ������ƿ�У�������Ӧ�÷���_______����ס����ҡ����С���ͬѧѡ�÷�̪��ָʾ��������жϴﵽ�ζ��յ㣺_______________________________��

��4�������±����ݣ����㱻���ռ���Һ�����ʵ���Ũ����______________ mol��L��1���ռ���Ʒ�Ĵ�����_______________��
�ζ����� | ������Һ ���(mL) | ������� | |
�ζ�ǰ�Ŀ̶�(mL) | �ζ���Ŀ̶�(mL) | ||
��һ�� | 10.00 | 0.40 | 10.50 |
�ڶ��� | 10.00 | 4.10 | 14.00 |
������ | 10.00 | 2.50 | 14.00 |
��5������ʵ�������Եζ��������ʲô�����(����ƫ������ƫ����������Ӱ����)
���۲���ʽ�ζ���Һ��ʱ����ʼƽ�ӣ��ζ��յ㸩�ӣ���ζ����______________��
���ζ�ǰ��ʽ�ζ��ܼ��촦�����ݣ��ζ���������ʧ����ζ����______________��
��ϴ�Ӻ���ƿδ�����ζ����______________��
����Ŀ������п���㷺Ӧ����ҽҩ�����ũҵ��������ҵ��������п����Ҫ�ɷ�ΪZnO������ZnSiO3��FeCO3��CuO�ȣ�����ZnSO47H2O��һ��������ͼ��

��1���������IJ�����____________��
��2������������ϡ����������ʱ���費��ͨ�����ˮ������Ŀ����______________��
��3���������У���pHԼΪ5.1����Һ�м��������أ�����Fe(OH)3��MnO(OH)2���ֳ������÷�Ӧ�����ӷ���ʽΪ____________________________________________��
��4���������У�����п�۵�������______________��
��5����֪����п���ܽ�����¶�֮��Ĺ�ϵ���±���
�¶�/�� | 0 | 20 | 40 | 60 | 80 | 100 |
�ܽ��/g | 41.8 | 54.1 | 70.4 | 74.8 | 67.2 | 60.5 |
������п��Һ�л������п�����ʵ�����Ϊ________________����ȴ�ᾧ�����ˡ���ɲ������ڼ�ѹ���������½��У�ԭ����_________________________________��
��6��ȡ28.70 g ZnSO47H2O��������ͬ�¶ȣ�ʣ�����������仯��ͼ��ʾ���������ݣ�680��ʱ���ù���Ļ�ѧʽΪ______��

a��ZnO b��Zn3O(SO4)2 c��ZnSO4 d��ZnSO4H2O