��Ŀ����
4�� �Ҷ����������ᣬ�����ǻ�ѧѧϰС���ͬѧ�Բ��ᾧ�壨H2C2O4•xH2O�����е�̽����ѧϰ
�Ҷ����������ᣬ�����ǻ�ѧѧϰС���ͬѧ�Բ��ᾧ�壨H2C2O4•xH2O�����е�̽����ѧϰ�Ĺ��̣�������벢Э������������ѧϰ����
����ͬѧ���о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O���е�xֵ��ͨ���������Ϻ������Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���ѧϰС���ͬѧ����˵ζ��ķ����ⶨxֵ��
�ٳ�ȡ2.520g�����ᾧ�壬�����Ƴ�100.00mLˮ��ҺΪ����Һ��
��ȡ25.00mL����Һ������ƿ�У��ټ���������ϡH2SO4��
����Ũ��Ϊ0.1000mol•L-1��KMnO4����Һ���еζ����ﵽ�յ�ʱ����20.00mL��
��1��д�����ᣨH2C2O4�������Ը��������Һ��Ӧ�Ļ�ѧ����ʽ��2��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O
��2���ζ�ʱ��������KMnO4��Һװ����ͼ�еļף���ס����ҡ����ζ����У�
��3����ʵ��ζ��ﵽ�յ�ı�־�ǵ��������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣮
��4��ͨ���������ݣ����x=2��
���ۣ������ζ��յ�ʱ���ӵζ��̶ܿȣ����ɴ˲�õ�xֵ��ƫС���ƫ����ƫС�����䡱����ͬ����
�����ζ�ʱ���õ�����KMnO4��Һ����ö�����Ũ�ȱ�С�����ɴ˲�õ�xֵ��ƫС��
���� ��1�����ᣨH2C2O4�������Ը��������Һ����������ԭ��Ӧ�����ᱻ�����ɶ�����̼��������ر���ԭ�������ӣ�
��2��KMnO4����ǿ�����ԣ��ḯʴ�ܣ�
��3��������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ㣻
��4���������ѧ����ʽ�����ݿɵó�x�����ζ��յ�ʱ���ӵζ��ܶ�������������������KMnO4��Һ�����ƫ���ɴ�����n��H2C2O4��ƫ����n��H2O��ƫС��xƫС���ݴ˷�����
��� �⣺��1�����ᣨH2C2O4�������Ը��������Һ����������ԭ��Ӧ�����ᱻ�����ɶ�����̼��������ر���ԭ�������ӣ���Ӧ�Ļ�ѧ����ʽΪ2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
�ʴ�Ϊ��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
��2����ΪKMnO4����ǿ�����ԣ��ḯʴ�ܣ���Ӧ����ʽ�ζ���ʢװ���ʴ�Ϊ���ף�
��3��������KMnO4��Һ��������ɫ��Ϊָʾ���жϵζ��յ�ʱ���ٵμ�KMnO4��Һʱ����Һ������ɫ��Ϊ��ɫ��
�ʴ�Ϊ�����������һ��KMnO4��Һʱ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ������ζ��յ㣻
��4���������ѧ����ʽ�����ݿ�֪��2KMnO4+5H2C2O4+3H2SO4=K2SO4+2MnSO4+10CO2��+8H2O��
2.520g�����ᾧ���к�H2C2O4�����ʵ���Ϊ��0.100 0 mol/L��20.00 mL��10-3 L/mL��$\frac{5}{2}$��$\frac{100ml}{25ml}$=0.0200 mol��0.02molH2C2O4������Ϊ0.02mol��90g/mol=1.8g������2.520g H2C2O4•xH2O��ˮ�����ʵ���Ϊ2.520g-1.8g=0.72g�������ʵ���=$\frac{0.72g}{18g/mol}$=0.04mol����x=2��
���ζ��յ�ʱ���ӵζ��ܶ�������������������KMnO4��Һ�����ƫ���ɴ�����n��H2C2O4��ƫ����n��H2O��ƫС��xƫС��
ͬ����������KMnO4��Һ����ö�����Ũ�ȱ�С�������������ƫ������xֵƫС��
�ʴ�Ϊ��2��ƫС��ƫС��
���� ���⿼���к͵ζ�ʵ�飬�Ѷ����У�ע�����ղ��Ậ���ļ��㷽�����к͵ζ��е����������ɽ��

 ѧ���������ν��Ͼ���ѧ������ϵ�д�
ѧ���������ν��Ͼ���ѧ������ϵ�д� Happy holiday���ּ��������ҵ�㶫���������ϵ�д�
Happy holiday���ּ��������ҵ�㶫���������ϵ�д�| A�� | ����0.1mol/L NaCl��Һʱ����û��ϴ���ձ��Ͳ���������������Һ���ʵ���Ũ��ƫ�� | |
| B�� | NH4NO3�ܽ����ȣ�������0.5mol/L H4NO3��Һʱֱ�ӽ��ܽ�����Һת�Ƶ�����ƿ�У���������Һ�����ʵ���Ũ��ƫ�� | |
| C�� | ����һ�����ʵ���Ũ����Һʱ�������������Ѿ����⣬��������Һ�����ʵ���Ũ��ƫ�� | |
| D�� | ����һ�����ʵ���Ũ����Һʱ���������в�С�ļ�ˮ�����̶��ߣ����̽�������ˮ��������������Һ�����ʵ���Ũ��ƫ�� |
| A�� | ú�� | B�� | Һ�� | C�� | �ȷ� | D�� | �Ҵ� |
| A�� |  | B�� |  | ||
| C�� | 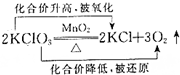 | D�� |  |
 һ���¶��£���1mol A��1mol B�������2L�����ܱ������У�������ӦA��g��+B��g��?xC��g��+D��s����t1ʱ�ﵽƽ�⣮��t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ���������������������C��Ũ����ʱ��ı仯��ͼ��ʾ������˵����ȷ���ǣ�������
һ���¶��£���1mol A��1mol B�������2L�����ܱ������У�������ӦA��g��+B��g��?xC��g��+D��s����t1ʱ�ﵽƽ�⣮��t2��t3ʱ�̷ֱ�ı䷴Ӧ��һ���������������������C��Ũ����ʱ��ı仯��ͼ��ʾ������˵����ȷ���ǣ�������| A�� | ��Ӧ����ʽ�е�x=1 | |
| B�� | t2ʱ�̸ı��������ʹ�ô��� | |
| C�� | t3ʱ�̸ı����������ȥ��������D | |
| D�� | t1��t3��÷�Ӧ��ƽ�ⳣ����Ϊ4 |
| A�� | ��������������ͨ�����������Һ�У�SO2+H2O+3ClO-�TSO42-+Cl-+2HClO | |
| B�� | �ð�ˮ�ܽ��Ȼ���������Ag++2 NH3•H2O�T[Ag��NH3��2]++2H2O | |
| C�� | �ö��Ե缫���MgCl2��Һ��2Cl-+2H2O$\frac{\underline{\;���\;}}{\;}$Cl2��+H2��+2OH- | |
| D�� | �����ʵ�����Ba��OH��2�루NH4��2Fe��SO4��2����Һ�з�Ӧ��Ba2++2OH-+2NH4++SO42-�TBaSO4��+2NH3•H2O |
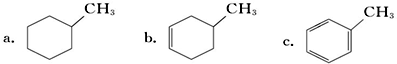 �����ڷ���������c������ĸ�������뱽�Ĺ�ϵ��ͬϵ�д�������巢����Ӧ�Ļ�ѧ����ʽ
�����ڷ���������c������ĸ�������뱽�Ĺ�ϵ��ͬϵ�д�������巢����Ӧ�Ļ�ѧ����ʽ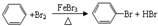 ��Ԥ��÷������ܣ���ܡ����ܡ����������෴Ӧ��
��Ԥ��÷������ܣ���ܡ����ܡ����������෴Ӧ�� ������ȡ����Ӧ�����ɱ���ȡ�����飺
������ȡ����Ӧ�����ɱ���ȡ�����飺 �����ڼӳɷ�Ӧ��
�����ڼӳɷ�Ӧ��