��Ŀ����
18��������A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A��һϵ�з�Ӧ���£����ַ�Ӧ������ȥ����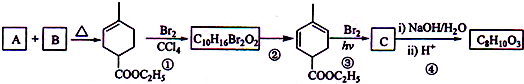
��֪��
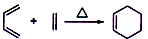
�ش��������⣺
��1��A�Ľṹ��ʽΪ����ѧ������2-��-1��3-����ϩ�������ϩ����
��2��B�ķ���ʽΪC5H8O2��
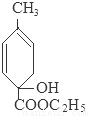
��3���ڵķ�Ӧ����ʽΪ
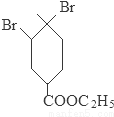 +2NaOH
+2NaOH
 +2NaBr+2H2O��
+2NaBr+2H2O����4���ٺ͢۵ķ�Ӧ���ͷֱ��Ǽӳɷ�Ӧ��ȡ����Ӧ��
��5��CΪ������������������-CH2-��д�����е�һ����Ӧ�Ļ�ѧ����ʽΪ
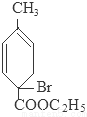 +2H2O
+2H2O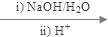
 +C2H5OH+HBr��
+C2H5OH+HBr����6��д������A��ͬ���칹���в����ۼ���ϩ��C=C=C���ṹ��Ԫ����״��ϩ����ͬ���칹��Ľṹ��ʽ
 ��
��
���� ������A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A�������ϩ��A��B��Ӧ���� �����������ϩ��
�����������ϩ�� �Ľṹ��ʽ֪��A��B�����˼ӳɷ�Ӧ��B�Ľṹ��ʽΪ��CH2=CHCOOCH2CH3��
�Ľṹ��ʽ֪��A��B�����˼ӳɷ�Ӧ��B�Ľṹ��ʽΪ��CH2=CHCOOCH2CH3�� ���巢���ӳɷ�Ӧ����
���巢���ӳɷ�Ӧ����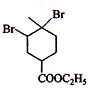 ��
�� ���������ƵĴ���Һ������ȥ��Ӧ����
���������ƵĴ���Һ������ȥ��Ӧ����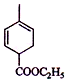 ��
�� ���巴Ӧ����C��CΪ�������������������Ǽ���C���������Ƶ�ˮ��Һ��Ӧ���ٺ��ᷴӦ����C8H10O3������C�Ľṹ��ʽΪ��
���巴Ӧ����C��CΪ�������������������Ǽ���C���������Ƶ�ˮ��Һ��Ӧ���ٺ��ᷴӦ����C8H10O3������C�Ľṹ��ʽΪ��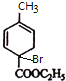 ������ʽΪC8H10O3�ṹ��ʽΪ
������ʽΪC8H10O3�ṹ��ʽΪ ��
��
��� �⣺������A�Ǻϳ���Ȼ�ĵ��壬����ʽΪC5H8��A�������ϩ��A��B��Ӧ���� �����������ϩ��
�����������ϩ�� �Ľṹ��ʽ֪��A��B�����˼ӳɷ�Ӧ��B�Ľṹ��ʽΪ��CH2=CHCOOCH2CH3��
�Ľṹ��ʽ֪��A��B�����˼ӳɷ�Ӧ��B�Ľṹ��ʽΪ��CH2=CHCOOCH2CH3�� ���巢���ӳɷ�Ӧ����
���巢���ӳɷ�Ӧ���� ��
�� ���������ƵĴ���Һ������ȥ��Ӧ����
���������ƵĴ���Һ������ȥ��Ӧ���� ��
�� ���巴Ӧ����C��CΪ�������������������Ǽ���C���������Ƶ�ˮ��Һ��Ӧ���ٺ��ᷴӦ����C8H10O3������C�Ľṹ��ʽΪ��
���巴Ӧ����C��CΪ�������������������Ǽ���C���������Ƶ�ˮ��Һ��Ӧ���ٺ��ᷴӦ����C8H10O3������C�Ľṹ��ʽΪ�� ������ʽΪC8H10O3�ṹ��ʽ
������ʽΪC8H10O3�ṹ��ʽ ��
��
��1��ͨ�����Ϸ���֪��A�������ϩ���ṹ��ʽΪ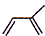 ����ѧ������2-��-1��3-����ϩ��
����ѧ������2-��-1��3-����ϩ��
�ʴ�Ϊ�� ��2-��-1��3-����ϩ��
��2-��-1��3-����ϩ��
��2��B�Ľṹ��ʽΪ��CH2=CHCOOCH2CH3���������ʽΪC5H8O2���ʴ�Ϊ��C5H8O2��
��3�� ���������ƵĴ���Һ������ȥ��Ӧ����
���������ƵĴ���Һ������ȥ��Ӧ����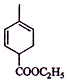 ����Ӧ����ʽΪ
����Ӧ����ʽΪ +2NaOH
+2NaOH
 +2NaBr+2H2O��
+2NaBr+2H2O��
�ʴ�Ϊ�� +2NaOH
+2NaOH
 +2NaBr+2H2O��
+2NaBr+2H2O��
��4��ͨ�����Ϸ���֪���ٺ͢۵ķ�Ӧ���ͷֱ��Ǽӳɷ�Ӧ��ȡ����Ӧ���ʴ�Ϊ���ӳɷ�Ӧ��ȡ����Ӧ��
��5��CΪ�������������������Ǽ���C���������Ƶ�ˮ��Һ��Ӧ���ٺ��ᷴӦ����C8H10O3��
��Ӧ����ʽΪ�� +2H2O
+2H2O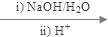
 +C2H5OH+HBr��
+C2H5OH+HBr��
�ʴ�Ϊ�� +2H2O
+2H2O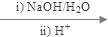
 +C2H5OH+HBr��
+C2H5OH+HBr��
��6�� ����ȥ�䱾������״��ϩ����ͬ���칹��Ϊ
����ȥ�䱾������״��ϩ����ͬ���칹��Ϊ ��
��
�ʴ�Ϊ�� ��
��
���� ���⿼���л�����ƶϣ���2����Ҫ�����Ϣ������ϩ������������л�����������������ע����дͬ���칹��ʱӦ����˳����д���簴��̼���칹��������λ���칹������칹����̼ͬ���Ķ�ϩ����Ȳ���������ѣ������������ǻ�ȩ����������칹����˳���칹�ȣ��籾�����һ�տ�����˼ά����Ӱ�죬ֻ���Ƕ�ϩ������ȥ����Ȳ�������ֻ�ܵõ�3��ͬ���칹�壬�Ӷ�����ͬ���칹���������Ϊ�״��㣮

| A�� | �������۷ų�H2����Һ�У�Al3+��K+��SO42-��Cl- | |
| B�� | 1.0mol•L-1��KNO3��Һ�У�Na+��Fe2+��Cl-��SO42- | |
| C�� | �ں�����Fe3+����Һ�У�NH4+��Na+��Cl-��HCO3- | |
| D�� | ˮ���������c��OH-��=10-12 mol•L-1����Һ�У�K+��Cu2+��SO42-��NO3- |
| A�� | ��0.1mol•L-1Na2C2O4��Һ�У�2c��Na+��=c��C2O42-��+c��HC2O4-��+c��H2C2O4�� | |
| B�� | ��10mL pH=12��NaOH��Һ�еμӵ����pH=2��CH3COOH��Һ��c��CH3COO-����c��Na+����c��OH-����c��H+�� | |
| C�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1��С�մ���Һ���ռ���Һ�������ϣ�c��Na+��+c��H+��=2c��CO32-��+c��OH-��+c��HCO3-�� | |
| D�� | Ũ�Ⱦ�Ϊ0.1 mol•L-1�����������Һ������������Һ��������c��SO42-��=c��Na+����c��NH4+����c��H+����c��OH-�� |
| A�� | ʮ���� | B�� | ʮ���� | C�� | ʮ���� | D�� | ʮ���� |

| A�� | ���ǻ���������Է�����ȥ��Ӧ��ȡ����Ӧ�����۷�Ӧ | |
| B�� | ���ӺͶ��ǻ���������ͬϵ�� | |
| C�� | ��ȩ����H2���ȵ������·�Ӧ�����Ҷ��� | |
| D�� | �ں˴Ź��������ж��ǻ�������Ӧ����6�����շ� |
| A�� |  �������ɳ�� | B�� | 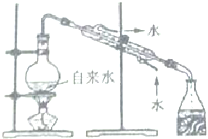 ʵ������ȡ����ˮ | ||
| C�� | 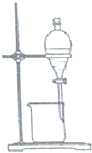 �Ҵ���ȡ��ˮ�еĵ� | D�� | 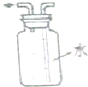 �ռ����� |
| A�� | ��0.1 molL-1SO2����Һ�У�Na+��Ba2+��Br-��Cl- | |
| B�� | �����������Ӧ�ų���������Һ�У�K+��Mg2+��NO${\;}_{3}^{-}$��SiO${\;}_{3}^{2-}$ | |
| C�� | $\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=10-12����Һ�У�K+��NH${\;}_{4}^{+}$��SO${\;}_{4}^{2-}$��NO${\;}_{3}^{-}$ | |
| D�� | ����ʹ��̪������ɫ��Һ�У�Na+��K+��ClO-��I- |
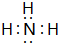 ��
��
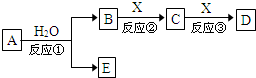 �ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E����ͼת����ϵ������������ͷ�Ӧ�����ԣ���
�ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E����ͼת����ϵ������������ͷ�Ӧ�����ԣ���