��Ŀ����
����Ŀ������ֲ���纣���������к��зḻ�ĵ�Ԫ�ء���Ԫ���Ե����ӵ���ʽ���ڡ�ʵ���ҴӺ�������ȡ����������£�
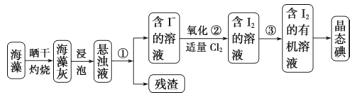
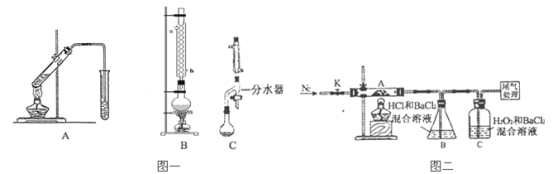
��1�� ָ���Ӻ�������ȡ I2 ��ʵ��������ƣ��� _______ ����_______��д���ڵ����ӷ���ʽ��______ ��
��2�� ��ȡ��Ĺ����У��ɹ�ѡ����л��ܼ���________��
A �ƾ� B ���Ȼ�̼ C ���� D ����
��3�� Ϊʹ������ I��ת��Ϊ����л���Һ��ʵ���������������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������ȱ�ٵ������� _____ ��
���𰸡����� ��ȡ��Һ 2I����Cl2=I2��2Cl�� B ��Һ©������ͨ©��
��������
����ɹ�����յõ��������к��е⻯�ص����ʣ�����ˮ�����ݺ���˵õ����е����ӵ���Һ���������Ϊ���ˣ���Һ��ͨ������������Cl2+2I-=2Cl-+I2���õ����ˮ��Һ��������Ϊ��ȡ�����л��ܼ���ȡ�õ�������л���Һ���������ɵõ��⣬�Դ������
(1)ָ���Ӻ�������ȡI2��ʵ��������ƣ��ٹ��ˣ�����ȡ�����̢����йط�Ӧ�����ӷ���ʽΪCl2+2I-=2Cl-+I2��
�ʴ�Ϊ�����ˣ���ȡ��Cl2+2I-=2Cl-+I2��
(2)��ȡ����ˮ�ֲ㣬�Ҳ�������Ӧ������ȡ��Ĺ����У��ɹ�ѡ����л��ܼ���B�����ƾ������������ˮ���ܣ�
�ʴ�Ϊ��B��
(3)Ϊʹ������Iת��Ϊ����л���Һ�����ܽ⡢���ˡ���������ȡ���룬ʵ���������ձ���������������ƿ���ƾ��ơ����ܡ�Բ����ƿ��ʯ�����Լ���Ҫ�ļг���������ȱ�ٵ������Ƿ�Һ©������ͨ©����
�ʴ�Ϊ����Һ©������ͨ©����