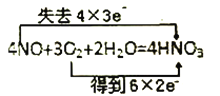
��Ŀ����
����Ŀ����ѧʵ����ʹ�õ�Ũ��������ʵ���������ͨ��Ϊ36.5%���ܶ�Ϊ1.25g/mL��
��1������������ʵ���Ũ��Ϊ__________mol/L��
��2������һ��0.2mol/L��ϡ����ĺ�����_________��ѡ����ĸ����
A��1Lˮ�к���0.2mol HCl
B��1L��Һ�к���0.1mol H+
C����7.1gHCl����1Lˮ����ɵ���Һ
D����100mL����Һ��ȡ��10mL��ʣ����Һ�����ʵ���Ũ����Ϊ0.2mol/L
��3��������ʵ���ҵ�Ũ����������500mL1 mol/L��ϡ���ᣬ�������в�����������д�йصĿո�
������Ͳ��ȡ________mL��Ũ�������ȷ��С�����һλ��
����ʢ����������ˮ���ձ��У���_________����ע��Ũ���
�۽�����ȴ�����µ�������Һ�ز�����ע��________mL������ƿ�У�
������������ˮϴ���ձ�2��3�Σ�����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У�
�ݼ���������ƿ�м�����ˮ��ֱ��Һ��ӽ��̶���1��2cm����
����___________________��μ�����ˮ��ʹ��Һ��Һ��ǡ����̶������У�
�߸Ǻ�����ƿ���������ߵ���ҡ�ȣ�
�ཫ��õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ��
��4�����ڲ���������������ʵ������������������Ƶ�������Һ�����ʵ���Ũ���к�Ӱ��?������ƫ��������ƫ����������Ӱ������գ�
������ƿ������ˮϴ�Ӻ������������ˮ_____________��
�ڶ���ʱ��ijͬѧ�۲�Һ��������ͼ��ʾ_____________��
��ҡ�Ⱥ�����Һ��Һ����ڿ̶��ߣ��ּ�ˮ���̶���____________��
���𰸡�12.5 D 40.0 �ձ��ڱ� 500 ��ͷ�ι� ��Ӱ�� ƫ�� ƫ��
��������
��1������c=![]() ���㣻
���㣻
��2���������ʵ���Ũ�ȵĺ��������
��3������ϡ��ԭ���������Ũ�����������ٸ������ƹ淶�IJ��������
��4�����ݲ������������ʵ����ʵ�������Һ�������ɵ�Ӱ�죬���c=![]() ����Ӱ������
����Ӱ������
(1)������������Ϊ36.5%���ܶ�Ϊ1.25g/mL��Ũ�������ʵ���Ũ��c=![]() =
=![]() mol/L=12.5mol/L���ʴ�Ϊ��12.5��
mol/L=12.5mol/L���ʴ�Ϊ��12.5��
(2)A��0.2mol/L��ϡ����ĺ�����1L��Һ�к���0.2mol HCl ������1Lˮ�к���0.2mol HCl����A����
B��1L��Һ�к���0.2mol HCl ���Ȼ�����ȫ���룬����0.2mol H+����B����
C��0.2mol/L��ϡ����ĺ�����1L��Һ�к���0.2mol HCl ����7.1gHCl����1Lˮ����ɵ���Һ�������1L����C����
D����Һ���о�һ�ԣ���100mL����Һ��ȡ��10mL��ʣ����Һ�����ʵ���Ũ����Ϊ0.2mol/L����D��ȷ��
��ѡD��
(3)��12.5mol/L��Ũ����������500mL1 mol/L��ϡ���ᡣ����Һϡ��ǰ���Ȼ�������ʵ������䣬��ҪŨ��������=![]() =0.04L=40.0mL��
=0.04L=40.0mL��
����ʢ����������ˮ���ձ��У����ձ��ڱ�����ע��Ũ����۽�����ȴ�����µ�������Һ�ز�����ע��500mL������ƿ�У�������������ˮϴ���ձ�2��3�Σ�����ϴ��ҺҲȫ��ת�Ƶ�����ƿ�У��ݼ���������ƿ�м�����ˮ��ֱ��Һ��ӽ��̶���1��2cm�������ý�ͷ�ι���μ�����ˮ��ʹ��Һ��Һ��ǡ����̶������У��߸Ǻ�����ƿ���������ߵ���ҡ�ȣ��ཫ��õ�ϡ���ᵹ���Լ�ƿ�У����ñ�ǩ���ʴ�Ϊ��40.0���ձ��ڱڣ�500����ͷ�ιܣ�
(4)������ƿ������ˮϴ�Ӻ������������ˮ����Ӱ�����ʵ����ʵ�������Һ���������Ũ��û��Ӱ�죬�ʴ�Ϊ����Ӱ�죻
�ڶ���ʱ����������ƿ�̶��ߣ�������Һ�����ƫС��Ũ��ƫ�ߣ��ʴ�Ϊ��ƫ�ߣ�
��ҡ�Ⱥ�����Һ��Һ����ڿ̶��ߣ��ּ�ˮ���̶��ߣ�������Һ�����ƫ��Ũ��ƫ�ͣ��ʴ�Ϊ��ƫ�͡�