��Ŀ����
�о�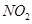 ��
��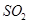 ��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��1�� ��ʹ
��ʹ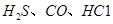 �������������ڶ����ⶨCO�ĺ�������֪��
�������������ڶ����ⶨCO�ĺ�������֪��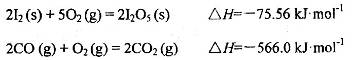
д��CO��g����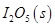 ��Ӧ����
��Ӧ����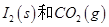 ���Ȼ�ѧ����ʽ��________________��
���Ȼ�ѧ����ʽ��________________��
��2��CO������ȼ�ϵ�أ���KOH��Һ������ʣ��������ֱ����CO�Ϳ��������������У�K+����_______��(���������)��������Ӧ����ʽΪ��___________________��
��3�����Ͱ��������������Ļ�ѧԭ���Dz��ð�ˮ���������е�SO2������һ��������
�����������ղ��ﷴӦ���ü������ŵ�����ܻ�������SO2�⣬���ܵõ�һ�ָ��Ϸ��ϡ�
�ٸø��Ϸ��Ͽ��ܵĻ�ѧʽΪ___________(д��һ�ּ���)��
������ˮ��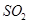 ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
ǡ����ȫ��Ӧ�������Σ����ʱ��Һ��________��(��ᡱ�)��
������������ʵĵ���ƽ�ⳣ�����£���ˮ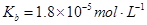

���������Һ��ͨ��________�����ʹ��Һ�����ԡ�(�SO2����NH3��)
��ʱ��Һ�� ________2�������������������
________2�������������������
��4��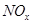 ����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�
����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�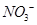 ��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
��ÿ��2�֣�
��1��5CO(g) + I2O5(s) = 5CO2 + I2(s) ?H= -1377.22kJ?mol?1
��2������O2 + 2H2O +4e? = 4OH?
��3���� (NH4)3PO4��(NH4)2HPO4��NH4H2PO4
�ڼ� �� SO2 >
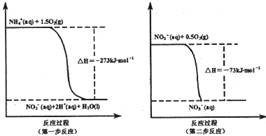
��4��3Fe2+ + NO3? + 4H+ =3Fe3+ +NO��+ 2H2O
���������������1����д����ѧ����ʽ�����������ʵ�״̬��Ȼ����ݸ�˹��������?H��
?H= �� ?H1 +
?H1 +  ?H2������������ݿɵô𰸡�
?H2������������ݿɵô𰸡�
��2��ԭ��ص������Һ�У����������������������ΪKOH��Һ������������ӦΪO2��H2O���������µõ�������OH?��
��3���ٷ�����Ӧ���������������Ӧ���������ᷴӦ�������������ͬ������(NH4)3PO4��(NH4)2HPO4��NH4H2PO4��
�ڰ�ˮ��SO2ǡ����ȫ��Ӧ����(NH4)2SO3��ˮ���Լ��ԡ�
����Ϊ������Һ�Լ��ԣ�����ͨ��SO2���к�OH?��ʹ��Һ�����ԣ����ݵ���غ��֪��[NH4+]+[H+]=[OH?]+2[SO32?]+[HSO3?]����Һ����[H+]=[OH?],��[NH4+]=2[SO32?]+[HSO3?]������[NH4+]/[SO32?]>2��
��4��������Ϣ�ҳ���Ӧ����������ƽ�ɵ����ӷ���ʽ��
���㣺���⿼���Ȼ�ѧ����ʽ����д��ԭ���ԭ���������ˮ�⡢����Ũ�ȱȽϺ����ӷ���ʽ����д��

��ҵ̼���ƣ�����ԼΪ98�����к���Ca2+��Mg2+��Fe3+��Cl����SO42�������ʣ��ᴿ������·��ͼ��ʾ��
��̼���Ƶı�����Һ�ڲ�ͬ�¶���������������ͼ��ʾ��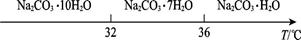
���й����ʵ��ܶȻ�����
| ���� | CaCO3 | MgCO3 | Ca��0H��2 | Mg��OH��2 | Fe��OH��3 |
| Ksp | 4.96��10��9 | 6.82��10��6 | 4.68��10��6 | 5.61��10��12 | 2.64��10��39 |
�ش��������⣺
��1������NaOH��Һʱ����Ӧ�����ӷ���ʽΪ ������Mg2+��Fe3+����Һ�еμ�NaOH��Һ�������ֳ�����������Һ��pH=8ʱ��c��Mg2+����c��Fe3+��= ��
��2����ĸҺ���г��˺���Na+��CO32���⣬������ �����ӡ�
��3�����˴ӡ���ɫ��ѧ���Ƕ����뽫��ĸҺ�������������߽���ѭ��ʹ�á����������ʵ�ʹ�ҵ�������Ƿ���У� ������С������С�������˵�����ɣ� ��
��4����֪��Na2CO3��10H2O��s��
 Na2CO3��s��+10H2O��g��
Na2CO3��s��+10H2O��g�� ="+532.36" kJ��mol��1
="+532.36" kJ��mol��1Na2CO3��10H2O��s��
 Na2CO3��H2O��s��+9H2O��g��
Na2CO3��H2O��s��+9H2O��g��  ="+473.63" kJ��mol��1
="+473.63" kJ��mol��1д��Na2CO3��H2O��ˮ��Ӧ���Ȼ�ѧ����ʽ�� ��
��Դ��ȱ���������ٵ��ش����⡣�״���һ�ֿ�������Դ�����й㷺�Ŀ�����Ӧ��ǰ������˼״�����Ϊ21���͵�����ȼ�ϡ�
��1����֪�ڳ��³�ѹ�£�
��2CH3OH(l)+3O2(g) 2CO2(g)+4H2O(g) ��H= ��1275.6 kJ��mol��1
��H2O(l) H2O(g) ��H="+" 44.0 kJ.mo��1
д����ʾ�״�ȼ���ȵ��Ȼ�ѧ����ʽ ��
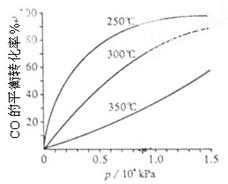
��2����ҵ����CO����ȼ�ϼ״���һ�������·�����Ӧ��CO(g)+2H2(g) CH3OH(g)��ͼ1��ʾ��Ӧ�������ı仯��ͼ2��ʾһ���¶��£������Ϊ2L���ܱ������м���4mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯ͼ��
CH3OH(g)��ͼ1��ʾ��Ӧ�������ı仯��ͼ2��ʾһ���¶��£������Ϊ2L���ܱ������м���4mol H2��һ������CO��CO��CH3OH(g)��Ũ����ʱ��仯ͼ��
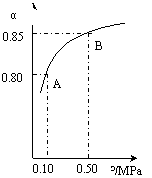
���ڡ�ͼ1���У����� ���a����b������ʾʹ���˴�����
�����жϸ÷�Ӧ�ڡ�ͼ2�������������Ƿ��Ѵﻯѧƽ��״̬�������� ����˫ѡ��
| A��������ѹǿ���� | B����ϵ���ܶȲ���ʱ��ı� |
| C��v��(H2)=2v��(CH3OH) | D��CO��H2�����ʵ����ıȲ���ʱ��ı� |
 CH3OH(g)�Ļ�ѧƽ�ⳣ��K= ��
CH3OH(g)�Ļ�ѧƽ�ⳣ��K= �������ڡ�ͼ3���л���ƽ��ʱ�״��ٷֺ����������꣩���¶ȣ������꣩�仯�����ߣ�Ҫ��ѹǿ��ͬ��2�����ߣ��������ϱ��P1��P2����P1<P2����
��3��CaSO4��һ�������ʣ���֪Ksp��CaSO4��=9.10��10��6���ֽ�c mol��L��1 CaCl2��Һ��2.00��10��2 mol��L��1 Na2SO4��Һ�����������ɳ�������c����Сֵ�� ���������3λ��Ч���֣���
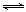 2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���8mol N2��9molO2��5min���ƽ��ʱNO���ʵ���Ϊ6mol������������µ�ƽ�ⳣ����д��������̣���
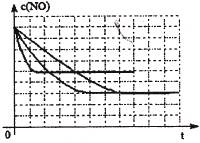
2NO(g)���ǵ�������β���к���NO��ԭ��֮һ��T ��ʱ����5L�ܱ������г���8mol N2��9molO2��5min���ƽ��ʱNO���ʵ���Ϊ6mol������������µ�ƽ�ⳣ����д��������̣��� 2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)���������ı����S1��S2 ����ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������
2CO2(g)+ N2(g) ��NO��Ũ�����¶�(T)�����������(S)��ʱ��(t)�ı仯���ߣ��ݴ��жϸ÷�Ӧ�ġ�H 0 (�����������������ȷ����)���������ı����S1��S2 ����ͼ�л���NO��Ũ����T1��S2 �����´ﵽƽ������еı仯���ߣ���ע��������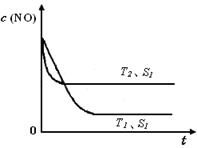
 2CO2+ N2 ��H
2CO2+ N2 ��H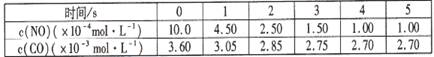

 H++ SO42-
H++ SO42-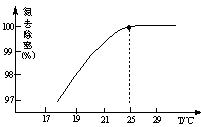
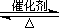 2SO3(g) ?H=-196��6 kJ��mol-1
2SO3(g) ?H=-196��6 kJ��mol-1 2NO2(g) ?H=-113��0 kJ��mol-1
2NO2(g) ?H=-113��0 kJ��mol-1