��Ŀ����
��1������Һ��ʯ��������Ҫ�ɷ�֮һ�Ƕ���(C4H10)����10 kg������ȫȼ�ղ����ɶ�����̼�����Һ̬ˮʱ���ų�������Ϊ5��105 kJ����д������ȼ�յ��Ȼ�ѧ����ʽ�� ����֪1molҺ̬ˮ����ʱ��Ҫ����44 kJ��������ӦC4H10(g)+6.5O2(g)=4CO2(g)+5H2O(g)�Ħ�H= ��
��2����ͬѧ�ö��������Ϊԭ������һȼ�յ�أ�ͨ�붡���һ��Ϊ ��������ϡ����Ϊ�������Һʱ����������ӦʽΪ ��
��3����֪:Fe(s) +1/2O2(g)=FeO(s) ��H=��272.0kJ��mol-1
2Al(s)+3/2O2(g)=Al2O3(s) ��H=��1675.7kJ��mol-1
Al��FeO�������ȷ�Ӧ���Ȼ�ѧ����ʽ�� ��
��4����֪��1 mol H��H����1 molN��H����1 molN��N���ֱ���Ҫ��������akJ��bkJ��ckJ����N2��H2��Ӧ����NH3���Ȼ�ѧ����ʽΪ ��

���������������1�����������������ʵ�������ϻ�ѧ����ʽ��Ӧ�����ʵ������㷴Ӧ�ų��������������Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ�д����������֪H2O(l)=H2O(g) ��H=44 kJ��mol-1,���ø�˹���ɼ���ɵã�
��2������--����ȼ�ϵ�ع���ʱ������ʧ���ӷ���������Ӧ����������ϡ����Ϊ�������Һʱ����ϵ缫��Ӧʽ����д���ɿɵã�
��3����������Ȼ�ѧ����ʽ��ϸ�˹����д�����Ȼ�ѧ��Ӧ����ʽ��������ʽ��-�١�3��2Al��s��+3FeO��s���TAl2O3��s��+3Fe��s����H=-859.7 kJ?mol-1��
(4)���ݡ�H=��Ӧ��ļ���֮�͡�������ļ���֮�Ϳ���ĺϳɰ���Ӧ���ʱ�Ϊ(3a+b-6c) kJ��mol-1�������Ȼ�ѧ����ʽ��д��������ע���ʾۼ�״̬�ͷ�Ӧ�ʱ�д����
���㣺�����Ȼ�ѧ����ʽ����д���ʱ�ļ����ȼ�ϵ�صȡ�

�Ͼ�ӡˢ��·��Ļ������ÿ�ʵ����Դ��������������Ⱦ���Ͼ�ӡˢ��·�徭������룬�ܵõ��ǽ�����ĩ�ͽ�����ĩ��
��1�����д���ӡˢ��·��ǽ�����ĩ�ķ����У������ϻ�������������� ������ĸ����
| A�����ѽ��γ�ȼ�� | B��¶����� | C����Ϊ�л����Ͻ������ϵ�ԭ�� | D��ֱ������ |
Cu(s)��2H��(aq)=Cu2��(aq)��H2(g) ��H=64.39kJ·mol��1
2H2O2(l)=2H2O(l)��O2(g) ��H=��196.46kJ·mol��1
H2(g)��1/2O2(g)=H2O(l) ��H=��285.84kJ·mol��1

�� H2SO4��Һ��Cu��H2O2��Ӧ����Cu2����H2O���Ȼ�ѧ����ʽΪ ��
��3����������������ͬ��ӡˢ��·��Ľ�����ĩ��10�GH2O2��3.0mol·L��1H2SO4�Ļ����Һ��������ò�ͬ�¶���ͭ��ƽ���ܽ����ʣ����±�����
| �¶ȣ��棩 | 20 | 30 | 40 | 50 | 60 | 70 | 80 |
| ͭƽ���ܽ����ʣ���10-3 mol·L-1·min-1�� | 7.34 | 8.01 | 9.25 | 7.98 | 7.24 | 6.73 | 5.76 |
���¶ȸ���40��ʱ��ͭ��ƽ���ܽ��������ŷ�Ӧ�¶����߶��½�������Ҫԭ���� ��
��4�����ᴿ���CuSO4��Һ�м���һ������Na2SO3��NaCl��Һ�����ȣ�����CuCl�������Ʊ�CuCl�����ӷ���ʽ�� ��
��5�� ��֪��ͬ�����£�
4Ca5(PO4)3F(s)+3SiO2(s)=6Ca3(PO4)2(s)+2CaSiO3(s)+SiF4(g) ����H1
2Ca3(PO4)2(s)+10C(s)=P4(g)+6CaO(s)+10CO(g)����H2
SiO2(s)+CaO(s)=CaSiO3(s) ����H3
4Ca5(PO4)3F(s)+21SiO2(s)+30C(s)=3P4(g)+20CaSiO3(s)+30CO(g)+SiF4(g) ��
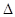 H
H
�á�H1����H2�͡�H3��ʾ
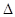 H��
H��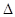 H= ��
H= ����6����֪1 g FeS2(s)��ȫȼ�����ɷų�7.1 kJ������FeS2ȼ�շ�Ӧ���Ȼ�ѧ����ʽΪ ��
 CH3OH(g)����ҵ��������CO����ȼ�ϼ״���
CH3OH(g)����ҵ��������CO����ȼ�ϼ״���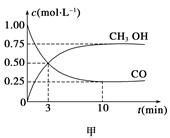
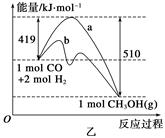
 �������____________��
�������____________�� 2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
2SO3(g)����Ӧ���̵������仯��ͼ��ʾ����֪1 mol SO2(g)����Ϊ1 mol SO3(g)�Ħ�H����99 kJ/mol��
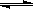 CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
CH3OH(g)��CO��ƽ��ת���ʣ��������¶ȡ�ѹǿ�Ĺ�ϵ��ͼ��ʾ��
 3N2��2X��4H2O
3N2��2X��4H2O  ��
��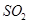 ��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣
��CO�ȴ�����Ⱦ����IJ���������������Ҫ���塣 ��ʹ
��ʹ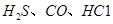 �������������ڶ����ⶨCO�ĺ�������֪��
�������������ڶ����ⶨCO�ĺ�������֪��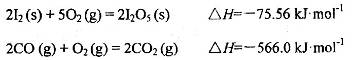
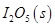 ��Ӧ����
��Ӧ����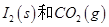 ���Ȼ�ѧ����ʽ��________________��
���Ȼ�ѧ����ʽ��________________��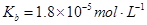

 ________2�������������������
________2�������������������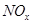 ����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�
����ǿ����Һ�����������Ρ������������£�FeSO4��Һ�ܽ�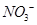 ��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��
��ԭΪNO��д���ù����в���NO��Ӧ�����ӷ���ʽ___________________________________��