��Ŀ����
����Ŀ����ϩ�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ش��������⣺
��1��������������������ϩ���Լ��� ��
A��ˮ
B��ϡ����
C����ˮ
D�����Ը��������Һ
��2����һ�������£���ϩ����ˮ��Ӧ�����л����������Ľṹ��ʽ�� ��
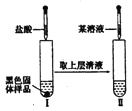
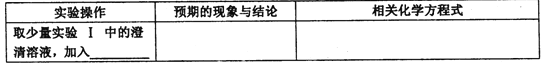
��3��ijͬѧ��ѧϰ����֪ʶ�����������ʵ�顣��������Ϊ��
�����Թ������2 mL A;
����һ���������״��ͭ˿���ھƾ��������м��ȣ�
��������ͭ˿����ʢ�У����Թ�������������Σ�
�������������ζ���۲�ͭ˿����ı仯��
�����������У�ͭ˿����ı仯�� ��д�������������з�����Ӧ�Ļ�ѧ����ʽ�� ��
��4��A��һ�������¿��Ա����������ᣬ�û�ѧ����ʽ˵�������̼�������ǿ���� ��
���𰸡���1��C D����2��CH3CH2OH����3���ɺ�ɫ��ɺ�ɫ��CH3CH2OH ��CuO![]() CH3CHO+Cu+H2O����4��CH3COOH+NaHCO3��CH3COONa+H2O+CO2����
CH3CHO+Cu+H2O����4��CH3COOH+NaHCO3��CH3COONa+H2O+CO2����
��������
�����������1������������ȶ�������ʹ��ˮ�����Ը��������Һ��ɫ����ϩ����̼̼˫������ʹ��ˮ�����Ը��������Һ��ɫ�����CD��ȷ����2����ϩ��ˮ�����ӳɷ�Ӧ��CH2=CH2��H2O��CH3CH2OH�����A�ṹ��ʽΪCH3CH2OH����3��2Cu��O2![]() 2CuO��CH3CH2OH��CuO
2CuO��CH3CH2OH��CuO![]() CH3CHO��Cu��H2O�������Ǻ�ɫ����ɫ����ɫ���䷴Ӧ����ʽΪ��CH3CH2OH ��CuO
CH3CHO��Cu��H2O�������Ǻ�ɫ����ɫ����ɫ���䷴Ӧ����ʽΪ��CH3CH2OH ��CuO![]() CH3CHO+Cu+H2O����4����������ǿ����ȡ��������CH3COOH+NaHCO3��CH3COONa+H2O+CO2����
CH3CHO+Cu+H2O����4����������ǿ����ȡ��������CH3COOH+NaHCO3��CH3COONa+H2O+CO2����

 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�