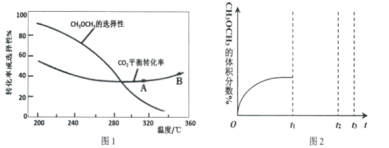
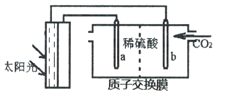
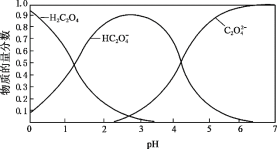
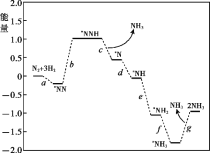
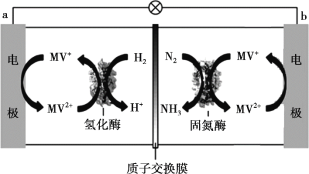
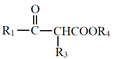��Ŀ����
����Ŀ��±���������������о��й㷺��Ӧ�ã��ش��������⣺
(1)���ȴ�������Ϊ�ܼ������з��ӽṹΪ�����������____________����ҵ�Ϸ�����Щ���ȴ�����ķ�����__________________��
(2)������������(CF3CHClBr)��һ����������д��������ͬ���칹��Ľṹ��ʽ��__________________________(�����������칹)��
(3)������ϩ�������г��������ϡ���ҵ����������ϩ��һ�ֹ���·�����£�
��ϩ![]() 1��2-��������
1��2-��������![]() ����ϩ
����ϩ![]() ������ϩ
������ϩ
��Ӧ�ٵĻ�ѧ����ʽ��______________________����Ӧ����Ϊ____________����Ӧ�ڵķ�Ӧ����Ϊ________________��
���𰸡����Ȼ�̼ ���� CF2BrCHFCl��CF2ClCHFBr��CHF2CFClBr H2C=CH2+Cl2��CH2ClCH2Cl �ӳɷ�Ӧ ��ȥ��Ӧ
��������
(1)���ӽṹΪ���������Ϊ���Ȼ�̼�����÷���ķ������룻
(2)CF3CHClBr��Ӧ��ͬ���칹���У��ɸ���±��ԭ�ӵ�λ�ò�ͬ�жϣ�
(3)��ϩ�����������ӳɷ�Ӧ����1��2-�������飬1��2-����������ȵ�480��530��������ȥ��Ӧ��������ϩ��CH2ClCH2Cl![]() H2C=CHCl +HCl������ϩ�����Ӿ۷�Ӧ�����ɾ�����ϩ��
H2C=CHCl +HCl������ϩ�����Ӿ۷�Ӧ�����ɾ�����ϩ��
(1)���ӽṹΪ����������ȴ�����Ϊ���Ȼ�̼����ҵ�Ϸ�����Щ���ȴ����飬�������ʵķе㲻ͬ�����÷���ķ������룻
(2)CF3CHClBr��Ӧ��ͬ���칹���У��ɸ���±��ԭ�ӵ�λ�ò�ͬ�жϣ���F����ͬһ��Cԭ���ϣ����ڵ�ͬ���칹����CF2BrCHFCl��CF2ClCHFBr��CHF2CFClBr ���֣�
(3)��ϩ�����������ӳɷ�Ӧ����1��2-�������飬��Ӧ�ķ���ʽΪH2C=CH2+Cl2��CH2ClCH2Cl��1��2-����������ȵ�480��530����������ϩ��������ȥ��Ӧ��

 Ӣ�ŵ��ϵ�д�
Ӣ�ŵ��ϵ�д� ������������Ծ�ϵ�д�
������������Ծ�ϵ�д� �������Ӳ�ϵ�д�
�������Ӳ�ϵ�д�����Ŀ����2L�ܱ������ڣ�800��ʱ��Ӧ��2NO(g)+O2(g)![]() 2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
2NO2(g)��ϵ�У�n(NO)��ʱ��ı仯�����
ʱ��(s) | 0 | 1 | 2 | 3 | 4 | 5 |
n(NO)(mol) | 0.020 | 0.01. | 0.008 | 0.007 | 0.007 | 0.007 |
��1����֪��K300����K350����д���÷�Ӧ��ƽ�ⳣ������ʽ��K=_________________�����ڸ÷�Ӧ������˵���У���ȷ����________��
A����H>0����S>0 B����H>0����S<0
C����H<0����S<0 D����H<0����S>0
��2����ͼ�б�ʾNO2�ı仯��������____________________����O2��ʾ��0-2s�ڸ÷�Ӧ��ƽ������v=_______________��
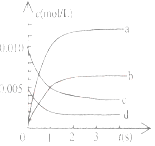
��3����˵���÷�Ӧ�Ѵﵽƽ��״̬����__________��
A��v(NO2)=2v(O2) B��������ѹǿ���ֲ���
C��v (NO)=2v��O2�� D���������ܶȱ��ֲ���
��4�����д�ʩ����ʹn(NO2)/n(NO)�������____��(����ĸ)
A�������¶� B���������
C�����ϳ���O2 D������He(g)��ʹ��ϵ��ѹǿ����