��Ŀ����
����Ŀ����ͭ���ǹ�ҵұ��ͭ��ԭ�ϣ���Ҫ�ɷ�Ϊ CuFeS2���Իش��������⣺
��1����̬��ԭ�Ӻ��������_____�ֲ�ͬ�˶�״̬��������ߵĵ�����ռ�ݵ�ԭ�ӹ����״Ϊ_________��
��2����̬Cuԭ�ӵļ۲�����Ų�ʽΪ_________��Cu��Zn�ĵڶ������ܴ�СI2(Cu) _________I2(Zn)(����>����<������=��)��
��3��SO2������Sԭ�ӵĹ���ӻ�����Ϊ_________�����ӿռ乹��Ϊ_________����SO2��Ϊ�ȵ�����ķ�����_________(дһ��)��
��4����ӽṹ�ǶȽ���H2SO3�����Ա�H2SO4��������ԭ��_________��
��5��Cu(CH3CN)4���İ���ͭ�����ȶ���������������ԭ����λ��Ϊ_________����λ������������������֮��Ϊ_________��
��6����þ�Ͻ���Ŀǰ�ѷ��ֵĴ����ܶ���ߵĴ������֮һ���侧���ṹ��ͼ��ʾ��

���þ��崢��ʱ��H2�����ھ��������ĺ��������λ�ã��������������H2����֮�����Ϊanm����þ�����ܶ�Ϊ_________g/cm3(�г��������ʽ)��
���𰸡�16 �Ĵ��� 3d104s1 �� sp2 V�� O3 ��������еķ��ǻ���ԭ������������࣬��������ԭ�����̬�ߣ����ڵ����H+ 4 5��2 ![]()
��������
(1)��Ԫ��Ϊ16��Ԫ�أ�������ߵĵ���Ϊ3p���ӣ�(2)CuΪ29��Ԫ�أ�ZnΪ30��Ԫ�أ�Cu�ĵڶ�������ʧȥ����3d������(3)SO2������Sԭ�ӵļ۲���Ӷ���=2+![]() (6-2��2)=3����ϵȵ�����ĸ�����(4)ͬһԪ�صIJ�ͬ�������У����ǻ���ԭ����Խ��������Խǿ��(5)Cu(CH3CN)4�е�����ԭ��ΪCu����λ��ΪCH3CN������������3��C-H��1��C-C��1��C��N��C��N�к���������(6)���ݾ���ṹ���������������H2����֮��ľ���Ϊ�������϶Խ��߳��ȵ�һ�룬������Mgԭ�Ӹ�����8��Feԭ�Ӹ���=8��
(6-2��2)=3����ϵȵ�����ĸ�����(4)ͬһԪ�صIJ�ͬ�������У����ǻ���ԭ����Խ��������Խǿ��(5)Cu(CH3CN)4�е�����ԭ��ΪCu����λ��ΪCH3CN������������3��C-H��1��C-C��1��C��N��C��N�к���������(6)���ݾ���ṹ���������������H2����֮��ľ���Ϊ�������϶Խ��߳��ȵ�һ�룬������Mgԭ�Ӹ�����8��Feԭ�Ӹ���=8��![]() +6��
+6��![]() =4�������仯ѧʽΪMg2Fe���ݴ˷������
=4�������仯ѧʽΪMg2Fe���ݴ˷������
(1)��Ԫ��Ϊ16��Ԫ�أ���̬��ԭ�Ӻ��������16�ֲ�ͬ�˶�״̬��������ߵĵ���Ϊ3p���ӣ�3p���ӵ�ԭ�ӹ����״Ϊ�Ĵ��Σ��ʴ�Ϊ��16���Ĵ��Σ�
(2)CuΪ29��Ԫ�أ���̬Cuԭ�ӵļ۲�����Ų�ʽΪ3d104s1��ZnΪ30��Ԫ�أ���̬Znԭ�ӵļ۲�����Ų�ʽΪ3d104s2��Cu�ĵڶ�������ʧȥ����3d���ӣ�ʧȥ���ȶ����ڶ������ܴ���Zn�ĵڶ������ܣ���I2(Cu)��I2(Zn)���ʴ�Ϊ��3d104s1������
(3)SO2������Sԭ�ӵļ۲���Ӷ���=2+![]() (6-2��2)=3������ӻ�����Ϊsp2�����ӵĿռ乹��ΪV�Σ���SO2��Ϊ�ȵ�����ķ�����O3���ʴ�Ϊ��sp2��V�Σ�O3��
(6-2��2)=3������ӻ�����Ϊsp2�����ӵĿռ乹��ΪV�Σ���SO2��Ϊ�ȵ�����ķ�����O3���ʴ�Ϊ��sp2��V�Σ�O3��
(4)ͬһԪ�صIJ�ͬ�������У����ǻ���ԭ����Խ��������Խǿ����������еķ��ǻ���ԭ������������࣬����H2SO4�����Դ���H2SO3�����ԣ��ʴ�Ϊ����������еķ��ǻ���ԭ������������࣬��������ԭ�����̬�ߣ����ڵ����H+��
(5)Cu(CH3CN)4�е�����ԭ��ΪCu����λ��Ϊ4����λ��ΪCH3CN������������3��C-H��1��C-C��1��C��N����5��������ֻ��C��N�к�����������2����������������������֮��5��2���ʴ�Ϊ��4��5��2��
(6)�辧���IJ���Ϊdnm���þ��崢��ʱ��H2�����ھ��������ĺ�����λ�ã���������������H2����֮��ľ���Ϊ�������϶Խ��߳��ȵ�һ�룬��anm=![]() ��d nm����d=
��d nm����d=![]() anm=
anm=![]() a��10-7cm���þ�����Mgԭ�Ӹ�����8��Feԭ�Ӹ���=8��
a��10-7cm���þ�����Mgԭ�Ӹ�����8��Feԭ�Ӹ���=8��![]() +6��
+6��![]() =4������Mg��Feԭ�Ӹ���֮��Ϊ8��4=2��1�������仯ѧʽΪMg2Fe���úϽ���ܶ�Ϊ=
=4������Mg��Feԭ�Ӹ���֮��Ϊ8��4=2��1�������仯ѧʽΪMg2Fe���úϽ���ܶ�Ϊ=![]() =
=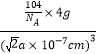 =
=![]() g/cm3���ʴ�Ϊ��
g/cm3���ʴ�Ϊ��![]() ��
��

 ״Ԫ����ϵ�д�
״Ԫ����ϵ�д� ͬ������ϵ�д�
ͬ������ϵ�д�����Ŀ����һ���¶��£�10 mL 0.40 mol/L H2O2��Һ�������ֽ⡣��ͬʱ�̲������O2�����(������Ϊ��״��)���±���
t/min | 0 | 2 | 4 | 6 | 8 | 10 |
V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ����(��Һ����仯���Բ���)(����)
A. 0��6 min��ƽ����Ӧ���ʣ�v(H2O2)��3.3��10��2 mol/(L��min)
B. 6��10 min��ƽ����Ӧ���ʣ�v(H2O2)<3.3��10��2 mol/(L��min)
C. ��Ӧ��6 minʱ��c(H2O2)��0.3 mol/L
D. ��Ӧ��6 minʱ��H2O2�ֽ���50%