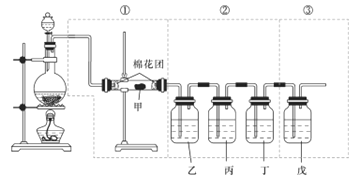
��Ŀ����
����Ŀ��ij�����Ĺ�ҵ��ˮ�к��д�����Fe2����SO42���ͽ϶��Cu2+��������Na����Ϊ�˼�����Ⱦ�����Ϊ���������ƻ��Ӹ÷�ˮ�л������������ͽ���ͭ�����������ͼ���ڷ���� ��������д�������ƣ�����Ҫ�ɷֵĻ�ѧʽ���������������ɻ�������������ͭ�ļ�ʵ �鷽����
��1��������������Ϊ__________������Ҫ�IJ�������Ϊ__________�����������ձ���
��2���Լ����Ļ�ѧʽΪ__________��������ѧ��Ӧ�����ӷ���ʽΪ__________��
��3��Ϊ��֤��Һ���к���SO42����ȡ��������Һ�����Թ��У��ȼ���____�ټ���__________�����۲쵽�а�ɫ��������˵����Һ���к���SO42����д��������Ӧ�����ӷ���ʽ______________________________��
���𰸡� ���� ©�� H2SO4 Fe+2H+=Fe2++H2���� ����ϡ���� ����BaCl2��Һ Ba2++SO42-=BaSO4��
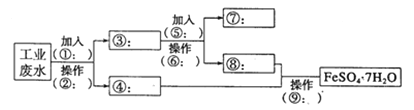
��������ij�����Ĺ�ҵ��ˮ�к��д�����Fe2����SO42���ͽ϶��Cu2+��������Na�����ȼӹ������٣����ˢڣ��õ�����ҺΪFeSO4����ΪCu�Ͷ��������������мӹ���ϡ����ݣ��������õ��Ģ�ΪCu����ΪFeSO4������1�������ڵ�����Ϊ���ˣ�����Ҫ�IJ�������Ϊ©�������������ձ�����2���Լ��ݵĻ�ѧʽΪH2SO4��������Ӧ�����ӷ���ʽΪFe+2H+��Fe2++H2������3��SO42-�ļ��飺�ȼ�����ϡ���ᣬ�������ټ��Ȼ�����Һ���а׳�����֤������SO42-����Ӧ�����ӷ���ʽΪBa2++SO42-��BaSO4����

 �߲������Ӧ��һ��ͨϵ�д�
�߲������Ӧ��һ��ͨϵ�д�����Ŀ��I������������(Ni2O3)��һ�ֻҺ�ɫ����ζ�й���Ŀ�״������ϸ��ĩ��������������ܵ�ء���ҵ���Խ�������������NiCl2���̶�����Ni2O3�Ĺ����������£�

�±��г�����ؽ������������������������pH(��ʼ������pH����������Ũ��Ϊ1.0 mol��L��1����)��
�������� | Fe(OH)3 | Fe(OH)2 | Al(OH)3 | Ni(OH)2 |
��ʼ������pH | 1.1 | 6.5 | 3.5 | 7.1 |
������ȫ��pH | 3.2 | 9.7 | 4.7 | 9.2 |
��1����Ϊ����߽��������Ͻ��������ʣ����������ʱ�ɲ�ȡ�Ĵ�ʩ�У��ʵ������¶ȣ����裬______�ȡ�
��������������Һ�к���Ni2����Cl��������������Fe2����Fe3����Al3���ȡ��ڳ���ǰ�����Na2CO3������ҺpH��ΧΪ______��
��2��������������Ni2O3�����ӷ���ʽΪ______��
��3����ҵ������Ϊ���������0.05 ~ 0.1 mol��L��1 NiCl2 ��Һ��һ����NH4Cl��ɵĻ����Һ���ɵõ��ߴ��ȡ����εij�ϸ���ۡ�����������һ��ʱ��NH4Cl��Ũ�ȶ���������Ч�ʼ����ijɷ��ʵ�Ӱ������ͼ��ʾ����NH4Cl��Ũ����ÿ���Ϊ______��

II��ú����Ȼ���Ĺ������̼�ͼ������

��4����֪��ӦI��C(s)��H2O(g)![]() CO(g)��H2(g) ��H����135 kJ��mol��1��ͨ����������벿��̼����ȼ�շ�Ӧ������������ת����ƽ���ƶ�ԭ��˵��ͨ�����������ã�______��
CO(g)��H2(g) ��H����135 kJ��mol��1��ͨ����������벿��̼����ȼ�շ�Ӧ������������ת����ƽ���ƶ�ԭ��˵��ͨ�����������ã�______��
��5���ټ��黯��ӦIV����֮ǰ��Ҫ�������ᷴӦIII��ú����ӦI��II��������к��������������壬�ֱ���H2S��_____��
�ڹ�ҵ�ϳ�����̼�����Һ�ѳ�H2S����õ�������ʽ�Σ��÷�Ӧ�����ӷ���ʽ��____��
��6��һ���������������Ϊ2L�ĺ����ܱ������г���1.2 mol CH4(g)��4.8 mol CO2(g)��������Ӧ��CH4 (g)��3CO2 (g)![]() 2H2O(g)��4CO(g) ��H��0��ʵ��������Ӧ���յ������ͼ�������������ʱ��仯������ͼ������ͼ����������������˷�Ӧ��
2H2O(g)��4CO(g) ��H��0��ʵ��������Ӧ���յ������ͼ�������������ʱ��仯������ͼ������ͼ����������������˷�Ӧ��![]() H��______��
H��______��