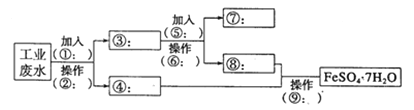
��Ŀ����
����Ŀ����NA���������ӵ�������ֵ������˵����ȷ����
A. ��״������22.4LHF�к��еķ�ԭ����ĿΪNA
B. 12gʯīϩ(����ʯī)�к��е���Ԫ������ΪNA
C. ��״������6.0gNO��2.24LO2��ϣ���������ķ�����ĿΪ0.2NA
D. ��ӦN2(g)+3H2(g)![]() 2NH3(g) ��H=-92.4kJ/mol�����ų�����4.62kJ����ת�Ƶĵ�����ĿΪ0.3NA
2NH3(g) ��H=-92.4kJ/mol�����ų�����4.62kJ����ת�Ƶĵ�����ĿΪ0.3NA
���𰸡�D
��������A. ��״���£��������Һ̬��22.4LHF�к��еķ�ԭ����Ŀ����NA����A����B. ʯīϩ(����ʯī)��ƽ��ÿ����Ԫ������2��̼ԭ�ӣ�12g��1molʯīϩ(����ʯī)�к���0.5mol��Ԫ������Ԫ������Ϊ0.5NA����B����C. ��״���£�6.0g��0.2molNO��2.24L��0.1molO2��ϣ�2NO+O2![]() 2NO2��2NO2
2NO2��2NO2![]() N2O4����������С��0.2mol������ķ�����ĿС��0.2NA����C����D. ÿ�ų�����92.4kJ��ת�Ƶ���6mol�����ų�����4.62kJ����ת�Ƶĵ�����ĿΪ0.3NA����D��ȷ����ѡD��
N2O4����������С��0.2mol������ķ�����ĿС��0.2NA����C����D. ÿ�ų�����92.4kJ��ת�Ƶ���6mol�����ų�����4.62kJ����ת�Ƶĵ�����ĿΪ0.3NA����D��ȷ����ѡD��

��ϰ��ϵ�д�
�����Ŀ