��Ŀ����
�ס��ҡ�����������ѧ��ѧ���������ʣ����мס��ҡ���������ͬһ��Ԫ�أ���һ���������ת���Ĺ�ϵ��ͼ��ʾ��

��ش��������⣺
��1������Ϊ���������;���Ľ������ʣ����������ҵ���Һû�еõ��ҵ��Σ����ҵĻ�ѧʽ����Ϊ ��д��һ�ּ��ɣ�����2.8g��ȫ������һ��Ũ�ȡ�200mL��HNO3��Һ�У��õ���״���µ�����1.12L����÷�Ӧ����Һ��pHΪ1������Ӧǰ����Һ����仯���Բ��ƣ���Ӧ�����Һ�����ܽ� g����
��2����ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣���Ӧ�ٵĻ�ѧ����ʽ ��
��3������Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ��������ˮ�е��ܶȻ�����ʽΪ ����Ӧ�۵����ӷ���ʽΪ ��
��4�����ס��ҡ�������Һ���Լ��ԣ�������Ϊҽ��������θ�����֢��ҩ�����������ʵ������Һͱ�����ˮ�γɻ����Һ����Һ�и�������Ũ���ɴ�С˳��Ϊ ����0.1mol��ͨ���ҵ�ϡ��Һ����ȫ��Ӧ�ų�a kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪ ��

��ش��������⣺
��1������Ϊ���������;���Ľ������ʣ����������ҵ���Һû�еõ��ҵ��Σ����ҵĻ�ѧʽ����Ϊ
��2����ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣���Ӧ�ٵĻ�ѧ����ʽ
��3������Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ��������ˮ�е��ܶȻ�����ʽΪ
��4�����ס��ҡ�������Һ���Լ��ԣ�������Ϊҽ��������θ�����֢��ҩ�����������ʵ������Һͱ�����ˮ�γɻ����Һ����Һ�и�������Ũ���ɴ�С˳��Ϊ
���㣺������ƶ�
ר�⣺
��������1������Ϊ���������;���Ľ������ʣ�ӦΪFe�����������ҵ���Һû�еõ��ҵ��Σ�˵����ˮ�⣬ӦΪFeCl3 ��Fe��NO3��3�����Ϊ���������ᣬ��ΪFeCl2������������
��2����ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣�ӦΪ���������������ת����ϵ��֪��Ϊ��������Ϊ��������Ϊ��������ΪNO��
��3������Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ��ӦΪNaOH����ת����ϵ��֪��Ϊ���Σ���Ϊ������������Ϊƫ�����ƣ�
��4�����ס��ҡ�������Һ���Լ��ԣ���Ϊҽ��������θ�����֢��ҩ�������ΪNaHCO3���ס��ҡ�����ת����ϵΪNaOH
Na2CO3
NaHCO3��
��2����ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣�ӦΪ���������������ת����ϵ��֪��Ϊ��������Ϊ��������Ϊ��������ΪNO��
��3������Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ��ӦΪNaOH����ת����ϵ��֪��Ϊ���Σ���Ϊ������������Ϊƫ�����ƣ�
��4�����ס��ҡ�������Һ���Լ��ԣ���Ϊҽ��������θ�����֢��ҩ�������ΪNaHCO3���ס��ҡ�����ת����ϵΪNaOH
| CO2 |
| CO2 |
���
�⣺��1����DΪ���������;���Ľ�������ΪFe����������B����Һû�еõ�B���Σ�˵�����ζ�Ӧ����Ϊ�ӷ����ᣬ��B�Ļ�ѧʽ����ΪFeCl3 ��Fe��NO3��3��ת����ϵ�ֱ�Ϊ��Cl2
FeCl3
FeCl2��HNO3
Fe��NO3��3
Fe��NO3��2��
��Ӧ����ҺΪ������������Ļ����Һ������Һ����3c��Fe3+��+c��H+��=c��NO3-����������Ԫ���غ���n��Fe��=n��Fe3+��=0.05mol������c��Fe3+��=
=0.25mol/L����Ӧ����ҺpHΪ1������c��H+��=0.1mol/L������c��NO3-��=0.25mol/L��3+0.1mol/L=0.85mol/L��
ԭ������ϡ���ᣬ����NO���ɵ�Ԫ���غ��֪nԭ����HNO3��=3n[Fe��NO3��3]+nʣ����HNO3��+n��NO��=0.05mol��3+0.1mol/L��0.2+0.05mol=0.22mol������Fe��NO3��2��NOʱԭ�����ܽ������࣬��3 Fe+8HNO3=3Fe��NO3��2+2 NO��+4H2O ��֪��0.22molHNO3����ܽ���
��0.22mol������Ϊ
��0.22mol��56g/mol=4.62g���ʻ����ܽ���4.64g-2.8g=1.82g��
�ʴ�Ϊ��FeCl3 ��Fe��NO3��3��1.82g��
��2����ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣�ӦΪ���������������ת����ϵ��֪��Ϊ��������Ϊ��������Ϊ��������ΪNO����Ӧ�ٵĻ�ѧ����ʽ4NH3+3O2
2N2+6H2O���ʴ�Ϊ��4NH3+3O2
2N2+6H2O��
��3������Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ��ӦΪNaOH����ת����ϵ��֪��Ϊ���Σ���Ϊ������������Ϊƫ�����ƣ�
����ˮ�е��ܶȻ�����ʽKsp=c��Al3+����c3��OH-������Ӧ�۵����ӷ���ʽΪAl3++3AlO2-+6H2O=4Al��OH��3����
�ʴ�Ϊ��Ksp=c��Al3+����c3��OH-����Al3++3AlO2-+6H2O=4Al��OH��3����
��4�����ס��ҡ�������Һ���Լ��ԣ���Ϊҽ��������θ�����֢��ҩ�������ΪNaHCO3���ס��ҡ�����ת����ϵΪNaOH
Na2CO3
NaHCO3���������ʵ�����Na2CO3��NaHCO3����ˮ�γɻ����Һ������CO32-����ˮ������HCO3-���ӣ���c��HCO3-����c��CO32-����Na2CO3��NaHCO3ˮ��ʼ��ԣ���c��OH-����c��H+����ˮ��ij̶�ֻ���ٲ��֣���c��CO32-����c��OH-��������˳��Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����
��0.1molCO2ͨ��Na2CO3��ϡ��Һ����ȫ��Ӧ�ų�a kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪCO2��g��+Na2CO3��aq��+H2O��l��=2NaHCO3��ag����H=20akJ/mol��
�ʴ�Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����CO2��g��+Na2CO3��aq��+H2O��l��=2NaHCO3��ag����H=20akJ/mol��
| Fe |
| Fe |
| Fe |
| Fe |
��Ӧ����ҺΪ������������Ļ����Һ������Һ����3c��Fe3+��+c��H+��=c��NO3-����������Ԫ���غ���n��Fe��=n��Fe3+��=0.05mol������c��Fe3+��=
| 0.05mol |
| 0.2L |
ԭ������ϡ���ᣬ����NO���ɵ�Ԫ���غ��֪nԭ����HNO3��=3n[Fe��NO3��3]+nʣ����HNO3��+n��NO��=0.05mol��3+0.1mol/L��0.2+0.05mol=0.22mol������Fe��NO3��2��NOʱԭ�����ܽ������࣬��3 Fe+8HNO3=3Fe��NO3��2+2 NO��+4H2O ��֪��0.22molHNO3����ܽ���
| 3 |
| 8 |
| 3 |
| 8 |
�ʴ�Ϊ��FeCl3 ��Fe��NO3��3��1.82g��
��2����ͨ������¼ס��ҡ��������������壬���ҺͶ�Ϊ��������Ҫ�ɷ֣�ӦΪ���������������ת����ϵ��֪��Ϊ��������Ϊ��������Ϊ��������ΪNO����Ӧ�ٵĻ�ѧ����ʽ4NH3+3O2
| ||
| ||
��3������Ϊ�������Ϊ�ȼҵ����Ҫ��Ʒ��ӦΪNaOH����ת����ϵ��֪��Ϊ���Σ���Ϊ������������Ϊƫ�����ƣ�
����ˮ�е��ܶȻ�����ʽKsp=c��Al3+����c3��OH-������Ӧ�۵����ӷ���ʽΪAl3++3AlO2-+6H2O=4Al��OH��3����
�ʴ�Ϊ��Ksp=c��Al3+����c3��OH-����Al3++3AlO2-+6H2O=4Al��OH��3����
��4�����ס��ҡ�������Һ���Լ��ԣ���Ϊҽ��������θ�����֢��ҩ�������ΪNaHCO3���ס��ҡ�����ת����ϵΪNaOH
| CO2 |
| CO2 |
��0.1molCO2ͨ��Na2CO3��ϡ��Һ����ȫ��Ӧ�ų�a kJ��������÷�Ӧ���Ȼ�ѧ����ʽΪCO2��g��+Na2CO3��aq��+H2O��l��=2NaHCO3��ag����H=20akJ/mol��
�ʴ�Ϊ��c��Na+ ����c��HCO3-����c��CO32-����c��OH-����c��H+����CO2��g��+Na2CO3��aq��+H2O��l��=2NaHCO3��ag����H=20akJ/mol��
���������⿼���Ϊ�ۺϣ��漰Ԫ�ػ�������ƶϡ�Ũ�ȵļ��������Ũ�ȵĴ�С�Ƚϵ����⣬��Ŀ����һ���Ѷȣ�����ʱע������ƶϵ�˼·�������ˮ����ɣ�

��ϰ��ϵ�д�
�����Ŀ
ij����M�Ľṹʾ��ͼΪ �������йظ����ӵ�˵���в���ȷ���ǣ�������
�������йظ����ӵ�˵���в���ȷ���ǣ�������
 �������йظ����ӵ�˵���в���ȷ���ǣ�������
�������йظ����ӵ�˵���в���ȷ���ǣ�������| A��xֵ��6�����ϵĿ��� |
| B��M���ӵķ��ſ�����O2-��Ne��Al3+�� |
| C��M���ӱ�ʾ���������л�ԭ����������N3- |
| D��ȡ����M���ӵ���Һ������ɫ��Ӧ����Ϊ��ɫ����M�ķ�����Na+ |
���й��������Ǻ����ǵ�˵���У�������ǣ�������
| A�����ǵķ���ʽ��ͬ |
| B�����ǵķ��ӽṹ��ͬ |
| C�����Dz���ͬ���칹�壬������ͬϵ�� |
| D�����ǿ���ˮ�����������Ǻ��� |
����ʱ�����и���Һ�У����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A������̼������Һ�У�c��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3�� |
| B��pH=2�Ĵ����У�c��H+��=0.02mol?L-1 |
| C��0.1mol?L-1�Ȼ����Һ�У�c��H+����c��OH-����c��Cl-����c��NH4+�� |
| D��pH=12��NaOH��Һ������0.01mol?L-1�Ȼ����Һ��Ϻ�������Һ�У�c��Na+��=c��NH4+�� |
�ݱ��������Ǻͽ��Ǵ������п��ܴ���һ�ַdz��������̬��������ֻ�����ᵼ������ЧӦ�����ĽṹʽΪ16O=C=18O����16O=C=18O��������
| A����16O=C=16O��Ϊͬ�������� |
| B����18O=C=18O�м�����ͬ�Ļ�ѧ���� |
| C����Na216O2��Ӧ���ɵ������к���18O |
| D�����������16O=C=16O��18O=C=18O������庬����ͬ��ԭ�Ӹ��� |
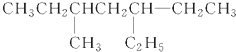 ��
��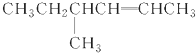 ��
��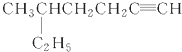 ��
�� ��
��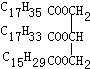 ij��֬���³�Һ̬������һ�ֳɷֵĽṹ��ʽ��ͼ��
ij��֬���³�Һ̬������һ�ֳɷֵĽṹ��ʽ��ͼ��