��Ŀ����
����ʱ�����и���Һ�У����ӵ����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������
| A������̼������Һ�У�c��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3�� |
| B��pH=2�Ĵ����У�c��H+��=0.02mol?L-1 |
| C��0.1mol?L-1�Ȼ����Һ�У�c��H+����c��OH-����c��Cl-����c��NH4+�� |
| D��pH=12��NaOH��Һ������0.01mol?L-1�Ȼ����Һ��Ϻ�������Һ�У�c��Na+��=c��NH4+�� |
���㣺����Ũ�ȴ�С�ıȽ�
ר�⣺�����ˮ��ר��
������A�����ݱ���̼������Һ�е������غ�����жϣ�
B����Һ��pH=-lgc��H+�����ݴ˼����pH=2�Ĵ�����Һ��������Ũ�ȣ�
C���Ȼ����Һ�У�笠����Ӻ�С�IJ���ˮ�⣬��Һ�������ӡ�笠�����Ũ��ԶԶ���������Ӻ�����������Ũ�ȣ�
D������Һ��Ӧ����һˮ�ϰ�����Һ��ʾ���ԣ���Һ��笠�����Ũ�Ⱥ�С��c��Na+����c��NH4+����
B����Һ��pH=-lgc��H+�����ݴ˼����pH=2�Ĵ�����Һ��������Ũ�ȣ�
C���Ȼ����Һ�У�笠����Ӻ�С�IJ���ˮ�⣬��Һ�������ӡ�笠�����Ũ��ԶԶ���������Ӻ�����������Ũ�ȣ�
D������Һ��Ӧ����һˮ�ϰ�����Һ��ʾ���ԣ���Һ��笠�����Ũ�Ⱥ�С��c��Na+����c��NH4+����
���
�⣺A������̼������Һ�У����������غ�ɵã�c��Na+��=2c��CO32-��+2c��HCO3-��+2c��H2CO3������A��ȷ��
B��pH=2�Ĵ����У�pH=-lgc��H+����������Ũ��Ϊ��c��H+��=10-2mol/L=0.01mol?L-1����B����
C.0.1mol?L-1�Ȼ����Һ�У���Һ��ʾ���ԣ���c��H+����c��OH-��������ֻ��������笠�����ˮ�⣬��Һ�������ӡ�笠�����Ũ��ԶԶ���������Ӻ�����������Ũ�ȣ�����Һ������Ũ�ȴ�С��ϵΪ��c��Cl-����c��NH4+����c��H+����c��OH-������C����
D��pH=12��NaOH��Һ��Ũ��Ϊ0.01mol/L��������0.01mol?L-1�Ȼ����Һ��Ϻ�������Һ�У�����������ǡ����笠����ӷ�Ӧ����һˮ�ϰ�����Һ��笠�����Ũ�Ⱥ�С��c��Na+��=c��Cl-����c��NH4+������D����
��ѡA��
B��pH=2�Ĵ����У�pH=-lgc��H+����������Ũ��Ϊ��c��H+��=10-2mol/L=0.01mol?L-1����B����
C.0.1mol?L-1�Ȼ����Һ�У���Һ��ʾ���ԣ���c��H+����c��OH-��������ֻ��������笠�����ˮ�⣬��Һ�������ӡ�笠�����Ũ��ԶԶ���������Ӻ�����������Ũ�ȣ�����Һ������Ũ�ȴ�С��ϵΪ��c��Cl-����c��NH4+����c��H+����c��OH-������C����
D��pH=12��NaOH��Һ��Ũ��Ϊ0.01mol/L��������0.01mol?L-1�Ȼ����Һ��Ϻ�������Һ�У�����������ǡ����笠����ӷ�Ӧ����һˮ�ϰ�����Һ��笠�����Ũ�Ⱥ�С��c��Na+��=c��Cl-����c��NH4+������D����
��ѡA��
���������⿼�����ж���Һ������Ũ�ȴ�С�ķ�������Ŀ�Ѷ��еȣ�ע�����ձȽ���Һ������Ũ�ȴ�С�ķ�����Ҫ��ѧ���ܹ����ݵ���غ㡢�����غ㡢�ε�ˮ��ԭ���ж���Һ������Ũ�ȴ�С��

��ϰ��ϵ�д�
 ������������Ӧ����ϵ�д�
������������Ӧ����ϵ�д� ͬ����չ�Ķ�ϵ�д�
ͬ����չ�Ķ�ϵ�д�
�����Ŀ
����������ָ��������Ŀһ������NA���ǣ�������
| A��11.2L�����ͳ����Ļ������������ԭ���� |
| B��1mol CaC2��������������� |
| C��1L 1mol/L CH3COOH��Һ�������������� |
| D��1mol Cl2�μӻ�ѧ��Ӧ��õĵ����� |
��a molNa2O2��b mol NaHCO3�����Ϻ����ܱ������м��ȵ�250�棬�����ַ�Ӧ�����ų�����Ϊ��������ʱ��a��b��ֵ�������ǣ�������
| A��1��1 | B��1��2 |
| C��2��3 | D��3��2 |
����˵����ȷ���ǣ�������
| A����Ȼ���к��д���������̬�Ĺ裬�����Ĺ辧����������������оƬ |
| B�������Ƶ�ˮ��Һ�׳�ˮ������������ľ�ķ����� |
| C��ˮ�ࡢ������ˮ�����ǹ�������Ʒ |
| D���������費���κ��ᷴӦ������ʯӢ������������ |
����ĸ����ǣ�������
| A������̼���⡢������Ԫ�ص��л��� |
| B������ͨʽCn��H2O��m�Ļ����� |
| C����������� |
| D��һ���Ƕ��ǻ�ȩ�����ǻ�ͪ�Լ���ˮ���������ǵ����� |
��NAΪ�����ӵ���������ֵ������˵����ȷ���ǣ�������
| A��1mol H2O2�к��еĵ�����Ϊ16NA |
| B����״���£�11.2L Br2�к�Br-Br����Ϊ0.5NA |
| C��1mol Fe������ϡHNO3��Ӧ��ת��3NA������ |
| D��1L 0.1 mol?L-1 NaHCO3��Һ�к���0.1NA��HCO3- |
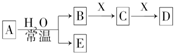 �ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X������ͼת����ϵ������������ͷ�Ӧ������ȥ�������ƶϲ���ȷ���ǣ�������
�ɶ�����Ԫ����ɵ���ѧ��������A��B��C��D��E��X������ͼת����ϵ������������ͷ�Ӧ������ȥ�������ƶϲ���ȷ���ǣ�������| A����A�ǵ��ʣ�B��D�ķ�Ӧ��OH-+HCO3-�TH2O+CO32-����E��һ�������Դ |
| B����DΪCO��C�ܺ�E��Ӧ����Aһ��ΪNa2O2 |
| C����X��Na2CO3��CΪ�����Լ��ķ��ӣ���Aһ������������D��E�ܷ�����Ӧ |
| D����DΪ��ɫ��������AĦ��������ȣ���Xһ�������� |

