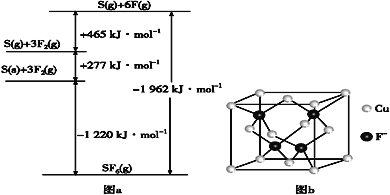
��Ŀ����
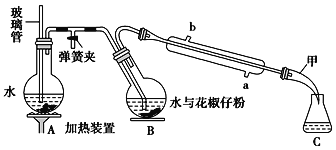
����Ŀ�����õ�ζ����������һ�ִӻ���������ȡ�Ļӷ����㾫�ͣ��������Ҵ������ѵ��л��ܼ���������ͼ��ʾװ�ô��������ѷۣ��������ᴿ�õ������͡�
ʵ�鲽�裺
(һ)��Aװ���е�Բ����ƿ��װ��![]() �ݻ���ˮ���Ӽ�����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
�ݻ���ˮ���Ӽ�����ʯ��ͬʱ����B�е�Բ����ƿ�м���20g�����ѷۺ�50mLˮ��
(��)����Aװ���е�Բ����ƿ�����д�����������ʱ�رյ��ɼУ���������
(��)�����Һ�м���ʳ�������ͣ�����15mL������ȡ2�Σ���������ȡ���Ѳ�ϲ�������������ˮNa2SO4����Һ���㵹��������ƿ�У�����û����͡�
��1��װ��A�в����ܵ�������___��װ��B��Բ����ƿ��б��Ŀ����___��
��2������(��)�У����۲쵽������������ɫ��״Һ�����ʱ����ֹͣ�����������ʱ�����в�����˳��Ϊ___(����)��
��ֹͣ���� �ڴ��ɼ� �۹ر�����ˮ
��3�������Һ�м���ʳ�ε�������___��������ˮNa2SO4��������___��
��4��ʵ���������ϡNaOH��Һ��ϴ�����ܣ���Ӧ�Ļ�ѧ����ʽΪ___��
(�������� ��ʾ)
��ʾ)
��5��Ϊ�ⶨ����������֬�ĺ�����ȡ20.00mL�����������Ҵ��У���80.00mL0.5mol/LNaOH���Ҵ���Һ�����裬��ַ�Ӧ����ˮ���200mL��Һ��ȡ25.00mL�����̪����0.1moI/L������еζ����ζ��յ���������20.00mL����û������к�����֬___g/L��
(�� �ƣ�ʽ����884)��
�ƣ�ʽ����884)��
���𰸡�ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ���� ��ֹ�ɽ����Һ�������������(��������) �ڢ٢� ���ͻ�������ˮ�е��ܽ�ȣ������ڷֲ� ��ȥ�������е�ˮ����� 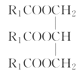 +3NaOH��3R1COONa+
+3NaOH��3R1COONa+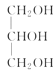 353.6
353.6
��������
��1��A�в�������������װ��A�в����ܵ�������ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ����װ��B��Բ����ƿ��б��Ŀ���Ƿ�ֹ�ɽ����Һ�������������(��������)���ʴ�Ϊ��ƽ����ѹ������رյ��ɼк�Բ����ƿ����ѹ����ֹ�ɽ����Һ�������������(��������)��
��2������(��)�У������Ͳ�����ˮ�����۲쵽������������ɫ��״Һ�����ʱ����ֹͣ�����������ʱ�����ɼУ�ֹͣ���ȣ��ر�����ˮ��
�ʴ�Ϊ�� �ڢ٢ۣ�
��3�������Ͳ�����ˮ�������Һ�м���ʳ�ε������ǽ��ͻ�������ˮ�е��ܽ�ȣ������ڷֲ㣻������ˮNa2SO4�������dz�ȥ�������е�ˮ�����ʴ�Ϊ�����ͻ�������ˮ�е��ܽ�ȣ������ڷֲ㣻��ȥ�������е�ˮ����
��4��ʵ���������ϡNaOH��Һ��ϴ�����ܣ�������Ϊ���࣬��NaOH��Һ��ˮ��Ϊ�����ƺʹ�����Ӧ�Ļ�ѧ����ʽΪ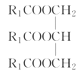 +3NaOH��3R1COONa+
+3NaOH��3R1COONa+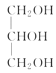 ��
��
�ʴ�Ϊ��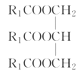 +3NaOH��3R1COONa+
+3NaOH��3R1COONa+ ��
��
��5������ζ�������NaOH���������n��NaOH��=n��HCl��=0.1mol��L��1��0.02L��![]() =0.016mol�������ˮ�ⷴӦ��n��NaOH��=0.5mol��L��1��0.08L=0.04mol-0.016mol=0.024mol���ʻ������е���֬���ʵ���Ϊ
=0.016mol�������ˮ�ⷴӦ��n��NaOH��=0.5mol��L��1��0.08L=0.04mol-0.016mol=0.024mol���ʻ������е���֬���ʵ���Ϊ![]() ��0.024mol=0.008mol����û������к�����֬Ϊ
��0.024mol=0.008mol����û������к�����֬Ϊ![]() =353.6g��L��1��
=353.6g��L��1��
�ʴ�Ϊ��353.6��

 Ӣ��СӢ������Ĭдϵ�д�
Ӣ��СӢ������Ĭдϵ�д� �����ҵ���������ͯ������ϵ�д�
�����ҵ���������ͯ������ϵ�д�����Ŀ�����и��������У�����ͼ����ʾ����ת����ϵ�Ҿ���һ������ʵ�ֵ���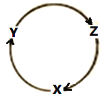
ѡ�� | X | Y | Z |
A | Na | NaOH | Na2O2 |
B | Fe | FeCl2 | Fe(OH)3 |
C | NO | NO2 | HNO3 |
D | Al | Al2O3 | Al(OH)3 |
A.AB.BC.CD.D