��Ŀ����
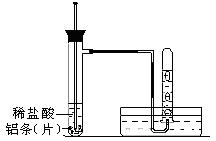
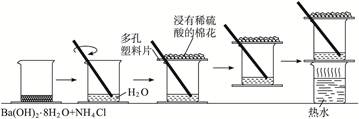
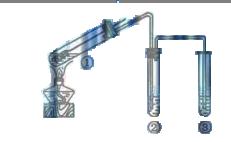
ijѧϰС��̽��Ũ��ϡ���������Ե����ǿ��������ͼװ�ý���ʵ��(�г���������ȥ)��ʵ�����Ũ�����ܽ�NO������NO2����ϡ���������NO���ɴ˵ó��Ľ�����Ũ�����������ǿ��ϡ���ᣮ

��ѡҩƷ��Ũ���ᡢ3mol��Lϡ���ᡢ����ˮ��Ũ���ᡢ����������Һ��������̼
��֪������������Һ����NO��Ӧ������NO2��Ӧ��2NO2+2NaOH=NaNO3+NaNO2+H2O ��1��װ�â��з�����Ӧ�����ӷ���ʽ�� ��
��2��װ�âڵ�Ŀ���� ��������Ӧ�Ļ�ѧ����ʽ�� ��
��3��ʵ��Ӧ�����к������ŷŵ������У�װ�âۡ��ܡ��ݡ�����ʢ�ŵ�ҩƷ������
��
��4��ʵ��ľ�������ǣ��ȼ���װ�õ������ԣ��ټ���ҩƷ��Ȼ����ɼУ�ͨ��
Ŀ���� ��
��5����С��ó��Ľ��������ݵ�ʵ�������� ��
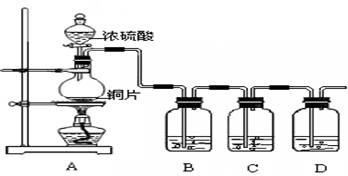
��1��Cu + 4H+ + 2NO3-(Ũ) = Cu2+ + 2NO2�� + 2H20��3�֣�
��2����NO2ת��ΪNO ��2�֣� 3NO2 + H2O = 2HNO3 + NO ��2�֣�
��3��3 mol��Lϡ���ᡢŨ���ᡢ����ˮ������������Һ(��1�֣���4��)
��4��CO2; ��1�֣�����װ���п�������������2�֣�
��5��װ�â���Һ���Ϸ�������Ϊ��ɫ��װ�â���Һ���Ϸ���������ɫ��Ϊ����ɫ��2�֣�
��

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
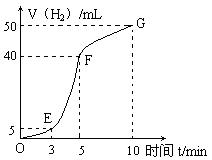
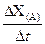 ��rX(A)��ʾ����A����������������Ũ�ȵȣ��ĸı�����ijѧϰС���ÿ�״��п��200mLϡ���ᷴӦ�о���ѧ��Ӧ���ʣ�ʵ��װ��ͼ����ͼ��
��rX(A)��ʾ����A����������������Ũ�ȵȣ��ĸı�����ijѧϰС���ÿ�״��п��200mLϡ���ᷴӦ�о���ѧ��Ӧ���ʣ�ʵ��װ��ͼ����ͼ��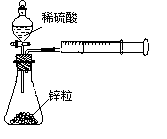
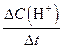
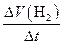
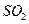 ����
���� ���ɣ�����м�ˮ���۲���ɫ
���ɣ�����м�ˮ���۲���ɫ