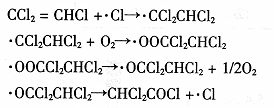��Ŀ����
(13��)������ijѧУ�о���ѧ��Ӧ�������仯�����ʵ�飺(���������������Ȼ�茶���ķ�Ӧ)
�±���ijѧ����������ʵ�鷽���Ͳ����б�������ʵ����ʵ�ͽ��ۣ�
(1)�������ʵ������ó���Ӧ��ʵ����������ϱ��С�
(2)�û�ѧ����ʽ��ʾ������ӦΪ_________________________________________________��

�±���ijѧ����������ʵ�鷽���Ͳ����б�������ʵ����ʵ�ͽ��ۣ�
| ʵ�鲽�� | ʵ������ | �ó����� |
| �������Ϻ������ò��������ٽ������� | �д̼�����ζ�������������������ʹʪ�����ɫʯ����ֽ���� | |
| ���ִ����ձ��²� | �о��ձ����� | |
| ���������ձ� | �ձ�����Ĵ��м���ˮ�IJ���Ƭ(��Сľ��)ճ�����ձ��ײ� | |
| ��ճ�в���Ƭ���ձ�����ʢ����ˮ���ձ���һ��������� | ����Ƭ���������ձ��ײ� | |
| ��Ӧ��������ձ��ϵĶ������QƬ���۲췴Ӧ�� | �����ɺ�״ | |
(2)�û�ѧ����ʽ��ʾ������ӦΪ_________________________________________________��
(1)
(2)Ba(OH)2��8H2O+2NH4Cl====BaCl2+2NH3��+10H2O
| | | ���� |
| | | ��NH3���� |
| | | ��Ӧ���� |
| | | ��Ӧ��������ʹ��ϵ�¶Ƚ��ͣ�ʹˮ��ɱ� |
| | | ���ڻ� |
| | | ��ˮ���� |
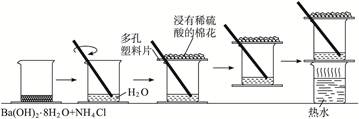
Ba(OH)2��NH4Cl�ķ�Ӧ�dz��������ȷ�Ӧ���ʱ������ҵ��µIJ���Ƭ�ᶳ�����ձ����¡����Ⱥ���Ƭ���ձ����룬��Ӧ��Ļ����������ˮ���ɶ��ɺ�״��

��ϰ��ϵ�д�
�����Ŀ