��Ŀ����
��14�֣�ij������ȤС����̽����������ķ�Ӧʵ���з�Ӧ��������Щ�����йء�����д���пհף�
��1��ʵ�鷴Ӧԭ���� ���� ��
��2��ʵ����Ʒ���Թܣ���ͨ�Թܺ;�֧�Թܸ�һ֧�������ӡ��齺�ܡ��������ܡ�
ˮ�ۡ���ͷ�ιܡ��¶ȼƣ�ϡ���ᣨ4mol/L����������Ƭ����ˮ��
��3��ʵ��װ�ã�ͼ������̨�ȼг�������ȥ��
��4��ʵ�鷽��
�ٰ���ͼ��ʾ���Ӻ�ʵ�����������װ�õ������ԣ�������������ǣ� ��
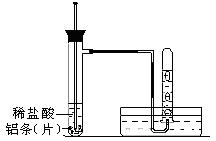
��ȡ3���������뵽�Թ��У����������� ��
����ʢ���������Թ��У�����2mLϡ���ᡣ
�ܲ����Թ�����Һ���¶ȡ�
���ռ���Ӧ������������
��5�����ݴ�������������
��ʵ�����������ȤС���Ա�����������������ʱ��������ͼ��
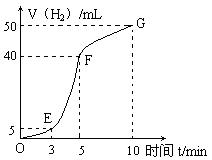
�����ж�OE��EF��FG�����ռ�����Ķ��١�
OE�� ��EF�� ��FG�� ��
д��������ʱ����ڲ������������ʴ�С�Ƚϣ���OE��EF��FG��ʾ�������ʴ�С�Ƚϣ� ��������ʱ��η�Ӧƽ�����ʱ仯��ԭ���� ��
��1��ʵ�鷴Ӧԭ���� ���� ��
��2��ʵ����Ʒ���Թܣ���ͨ�Թܺ;�֧�Թܸ�һ֧�������ӡ��齺�ܡ��������ܡ�
ˮ�ۡ���ͷ�ιܡ��¶ȼƣ�ϡ���ᣨ4mol/L����������Ƭ����ˮ��
��3��ʵ��װ�ã�ͼ������̨�ȼг�������ȥ��
��4��ʵ�鷽��
�ٰ���ͼ��ʾ���Ӻ�ʵ�����������װ�õ������ԣ�������������ǣ� ��

��ȡ3���������뵽�Թ��У����������� ��
����ʢ���������Թ��У�����2mLϡ���ᡣ
�ܲ����Թ�����Һ���¶ȡ�
���ռ���Ӧ������������
��5�����ݴ�������������
��ʵ�����������ȤС���Ա�����������������ʱ��������ͼ��

�����ж�OE��EF��FG�����ռ�����Ķ��١�
OE�� ��EF�� ��FG�� ��
д��������ʱ����ڲ������������ʴ�С�Ƚϣ���OE��EF��FG��ʾ�������ʴ�С�Ƚϣ� ��������ʱ��η�Ӧƽ�����ʱ仯��ԭ���� ��
��1��2Al��6HCl��2AlCl3��3H2�� ��2�֣�
��4���ٽ������ܿڷ���ˮ�۵�ˮ�У�������ס��֧�Թ�һ����������������������ð�����ƿ��ֺ���������һ��ˮ����������ˮ���ڽϳ�ʱ���ڲ����䣬��֤����װ�����������á���2�֣�
��������ȡ������Ƭ����������б���Թܿ��ڣ�Ȼ���Թܻ���ֱ��������ʹ������Ƭ�����䵽�Թܵײ��� ��2�֣�
��5��5mL�� 35mL��10mL����ÿ��1�֣� EF>FG>OE��2�֣���OE�����ڷ�Ӧ�¶Ƚϵͣ����Է�Ӧ���ʽ��������ڸ÷�Ӧ�Ƿ��ȷ�Ӧ�����ŷ�Ӧ�Ľ��У��¶������ߣ�����EF�η�Ӧ���ʽϴ�FG����Ȼ�¶Ƚϸߣ������ŷ�Ӧ�Ľ��У�����Ũ����С�����Է�Ӧ�����ּ�С����3�֣�
��4���ٽ������ܿڷ���ˮ�۵�ˮ�У�������ס��֧�Թ�һ����������������������ð�����ƿ��ֺ���������һ��ˮ����������ˮ���ڽϳ�ʱ���ڲ����䣬��֤����װ�����������á���2�֣�
��������ȡ������Ƭ����������б���Թܿ��ڣ�Ȼ���Թܻ���ֱ��������ʹ������Ƭ�����䵽�Թܵײ��� ��2�֣�
��5��5mL�� 35mL��10mL����ÿ��1�֣� EF>FG>OE��2�֣���OE�����ڷ�Ӧ�¶Ƚϵͣ����Է�Ӧ���ʽ��������ڸ÷�Ӧ�Ƿ��ȷ�Ӧ�����ŷ�Ӧ�Ľ��У��¶������ߣ�����EF�η�Ӧ���ʽϴ�FG����Ȼ�¶Ƚϸߣ������ŷ�Ӧ�Ľ��У�����Ũ����С�����Է�Ӧ�����ּ�С����3�֣�
��

��ϰ��ϵ�д�
�����Ŀ