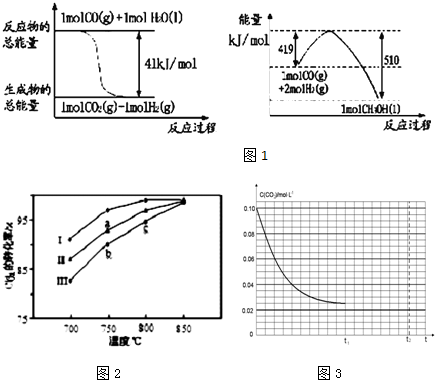
��Ŀ����
16��������Һ�У��й����ʵ���Ũ�ȹ�ϵ��ȷ���ǣ�������| A�� | ����������Һ�еμ�ϡ����õ���pH=5�Ļ����Һ��c��Na+ ��=c��NO3 - �� | |
| B�� | 1.0 mol•L-1Na2CO3 ��Һ��c��OH-��=c��HCO3-��+c��H+�� | |
| C�� | pH=8.0��KHS��Һ�У�c��K+����c��HS-����c��OH-����c��S2-����c��H+�� | |
| D�� | ��0.2 mol•L-1��ijһԪ��HA��Һ��0.1 mol•L-1NaOH��Һ�������Ϻ���ҺpH����7����Ӧ��Ļ��Һ�У�2c��OH-��+c ��A-��=2c��H+��+c��HA�� |
���� A�����������غ��жϣ�
B�����������غ��жϣ�
C��KHS��ҺpH=8��˵��HS-ˮ��̶ȴ��ڵ���̶ȣ�ע��ˮ�ĵ��룻
D����0.2 mol•L-1��ijһԪ��HA��Һ��0.1 mol•L-1NaOH��Һ�������Ϻ�HA��NaA��Ũ����ȣ���ҺpH����7��˵��A-ˮ��̶ȴ���HA����̶ȣ���������غ㡢����غ���
��� �⣺A���������������Ӧ�������Ƿ�������ᣬ������c��Na+��=c��NO3-������A��ȷ��
B��Na2CO3 ��Һ�д��������غ㣬Ϊc��OH-��=c��HCO3-��+c��H+��+2c��H2CO3������B����
C��KHS��ҺpH=8��˵��HS-ˮ��̶ȴ��ڵ���̶ȣ�����ˮ�ĵ��룬��c��H+����c��S2-������C����
D����0.2mol•L-1��ijһԪ��HA��Һ��0.1mol•L-1��NaOH��Һ�������ϣ�HA��������Ϻ���Һ��pH����7��˵��A-ˮ��̶ȴ���HA�ĵ���̶ȣ���Һ���ڵ���غ㣺c��OH-��+c��A-��=c��H+��+c��Na+�������������غ㣺c��A-��+c��HA��=2c��Na+����������ʽ�ɵ�2c��OH-��+c��A-��=2c��H+��+c��HA������D��ȷ��
��ѡAD��
���� �����ۺϿ�������ϵĶ����жϺ�����Ũ�ȵĴ�С�Ƚ����⣬��Ŀ�Ѷ��еȣ�������ע�����غ�������غ�����ã�

| A�� |  ��a�Ʊ����ռ����� | B�� |  ��b�Ʊ���������Ȳ | ||
| C�� |  ��c����ˮ�õ���ˮ | D�� |  ��d����Na2CO3��Һ��CH3COOC2H5 |
| A�� | 1��1 | B�� | 2��3 | C�� | 3��5 | D�� | 6��7 |
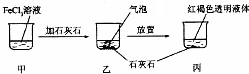
| A�� | ��Ӧ�����в�����������CO2 | |
| B�� | ����Һ��ɲ����������ЧӦ�� | |
| C�� | ��������Һ����ı仯���ձ���c��Cl- ���������仯 | |
| D�� | ���ڱ��м���������ᣬ��ַ�Ӧ��������Һ��������ͬ |
| A�� |  ��ͼ��ʾװ�����ʵ�����ư��� | |
| B�� | 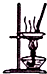 ��ͼ��ʾװ������FeCl3��Һ�Ʊ���ˮFeCl3 | |
| C�� | 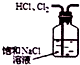 ��ͼ��ʾװ�ó�ȥHCl�к��е�����Cl2 | |
| D�� |  ��ͼ��ʾװ�ÿ����ڷ���������̺Ͷ��Ȼ��� |
| A�� | $\frac{11.2X}{5A}$mol-1 | B�� | $\frac{5A}{11.2X}$mol-1 | C�� | $\frac{22.4A}{5X}$mol-1 | D�� | $\frac{22.4X}{5A}$mol-1 |
| ѡ�� | ʵ�� | ���� | ���� |
| A | ͭƬ����Ũ������ | ��������ɫ���� | Ũ�������ǿ������ |
| B | ��һС���Ʒ����Ҵ��� | ������������ | ���Ҵ��к��н϶�ˮ |
| C | ��CuSO4��Һ��ͨ��H2S���� | ���ֺ�ɫ���� | H2S�����Ա�H2SO4ǿ |
| D | ��AgCl����Һ�еμ�KI��Һ | ��ɫ�������ɫ | AgI���ܽ�ȴ���AgCl |
| A�� | A | B�� | B | C�� | C | D�� | D |
| ��Һ | a | b | c | d | e |
| ���� | CH3COONa | Na2CO3 | NaClO | NaCN | C6H5ONa |
| pH | 8.8 | 11.6 | 10.3 | 11.1 | 11.3 |
��1������c��d��Һ�������ʶ�Ӧ����������ǿ��ΪHClO��HCN���û�ѧʽ��ʾ����
��2����ϱ������ݷ�����������������a��ˮ�ĵ���̶�Ӱ����ͬ����B��
A��pH=8.8��NaOH��Һ
B��pH=5.2��NH4Cl��Һ
C��pH=5.2������
D.0.2mol•L-1��NaCN��Һ
��3�������ӷ���ʽ��ʾc��Һ�ʼ��Ե�ԭ����ClO-+H2O?HClO+OH-��
��4�������b��Һ���뵽����ͬ���ʵ�����BaCl2��ZnCl2�Ļ��ϡ��Һ�У����������ij�����ZnCO3[��֪K��BaCO3��=5.1��10-9��K��ZnCO3��=1.4��10-11]��
��5��Ũ��Ϊ0.1mol•L-1��e������Ũ���ɴ�С��˳��Ϊc��Na+����c��C6H5O?����c��OH-����c��H+����
��6��100��ʱ��ˮ��c��H+��=10-6mol•L-1���ڴ��¶���ijŨ�ȵ�d��Һ��pH=n����c��H+��+c��HCN��=10n-12mol•L-1 ��