��Ŀ����
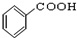
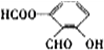
13���ҹ��ڶ�������֤���õ��Ǿ�����ɫ�������ܵ�PETG�²��ϣ�PETG�²��Ͽ��Ի��������ã����Ҷ��ܱ����������κ���Ⱦ��PETG�Ľṹ��ʽ��ͼ1��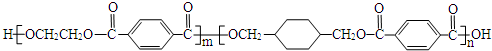
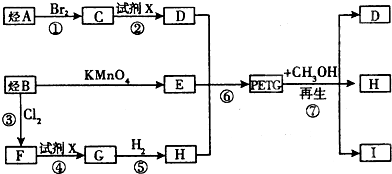
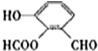
���ֲ��Ͽɲ�����ͼ2��ʾ�ĺϳ�·��
��֪��
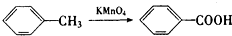
��RCOORl+R2OH��RCOOR2+R1OH ��R��R1��R2��ʾ������
�Իش��������⣺
��1����Ӧ�۵������ǹ��գ��ߵķ�Ӧ������ȡ����Ӧ��
��2���ϳ�ʱӦ���Ƶĵ�������ʵ�����n��H����n��E����n ��D��=n����m+n����m����m��n��ʾ����
��3��д����Ӧ�ڵĻ�ѧ����ʽ��CH2Br-CH2Br+2H2O$��_{��}^{NaOH}$HOCH2-CH2OH+2HBr
��4����֪D��E�ɺϳɵ��ڣ�д����Ӧ�Ļ�ѧ����ʽ��

��5��д��ͬʱ������������Ҫ���E������ͬ���칹��Ľṹ��ʽ
 ��
�� ��
��
��
�ٸ�ͬ���칹��ı��������ڵ�����̼ԭ���϶�����ȡ������
�ڸ�ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ��
��6��д��I�Ľṹ��ʽ��
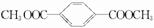 ��
��
���� ����PETG�Ľṹ��ʽ֪��PETG���� ��HOCH2CH2OH��
��HOCH2CH2OH�� ���ֵ���ͨ�����۷�Ӧ�õ���һ�ָ߾��PETG�ͼ״���Ӧ����D��I��H������RCOOR1+R2OH��RCOOR2+R1OH֪��D��H�Ǵ�����E��
���ֵ���ͨ�����۷�Ӧ�õ���һ�ָ߾��PETG�ͼ״���Ӧ����D��I��H������RCOOR1+R2OH��RCOOR2+R1OH֪��D��H�Ǵ�����E�� ��
��
B������ط�Ӧ���� ������B��
������B�� ��
�� ����������ȡ����Ӧ����F
����������ȡ����Ӧ����F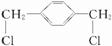 ��
�� ��X��Ӧ����G��G��Ӧ����H������H��
��X��Ӧ����G��G��Ӧ����H������H��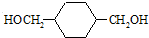 ����G��
����G�� ����D��HOCH2CH2OH����A��CH2=CH2����ϩ���巢���ӳɷ�Ӧ����CΪCH2BrCH2Br��PETG�ͼ״���Ӧ����D��I��H����������Ϣ֪��I��
����D��HOCH2CH2OH����A��CH2=CH2����ϩ���巢���ӳɷ�Ӧ����CΪCH2BrCH2Br��PETG�ͼ״���Ӧ����D��I��H����������Ϣ֪��I�� ���ݴ˷������
���ݴ˷������
��� �⣺����PETG�Ľṹ��ʽ֪��PETG���� ��HOCH2CH2OH��
��HOCH2CH2OH�� ���ֵ���ͨ�����۷�Ӧ�õ���һ�ָ߾��PETG�ͼ״���Ӧ����D��I��H������RCOOR1+R2OH��RCOOR2+R1OH֪��D��H�Ǵ�����E��
���ֵ���ͨ�����۷�Ӧ�õ���һ�ָ߾��PETG�ͼ״���Ӧ����D��I��H������RCOOR1+R2OH��RCOOR2+R1OH֪��D��H�Ǵ�����E�� ��
��
B������ط�Ӧ���� ������B��
������B�� ��
�� ����������ȡ����Ӧ����F
����������ȡ����Ӧ����F ��
�� ��X��Ӧ����G��G��Ӧ����H������H��
��X��Ӧ����G��G��Ӧ����H������H�� ����G��
����G�� ����D��HOCH2CH2OH����A��CH2=CH2����ϩ���巢���ӳɷ�Ӧ����CΪCH2BrCH2Br��PETG�ͼ״���Ӧ����D��I��H����������Ϣ֪��I��
����D��HOCH2CH2OH����A��CH2=CH2����ϩ���巢���ӳɷ�Ӧ����CΪCH2BrCH2Br��PETG�ͼ״���Ӧ����D��I��H����������Ϣ֪��I�� ��
��
��1��ͨ�����Ϸ���֪����Ӧ�۵������ǹ��գ���Ӧ�ߵ�������ȡ����Ӧ��
�ʴ�Ϊ�����գ�ȡ����Ӧ��
��2���ɾۺ���PETG��֪���ϳ�ʱӦ���Ƶĵ�������ʵ�����n��H����n��E����n��D��=n����m+n����m��
�ʴ�Ϊ��n����m+n����m��
��3��ͨ�����Ϸ���֪���ڵķ�Ӧ����ʽΪ��CH2Br-CH2Br+2H2O$��_{��}^{NaOH}$HOCH2-CH2OH+2HBr���ʴ�Ϊ��CH2Br-CH2Br+2H2O$��_{��}^{NaOH}$HOCH2-CH2OH+2HBr��
��4��D��E�ڴ��������¿�����һ�־�����ά--���ڣ��÷�Ӧ�Ļ�ѧ����ʽΪ ��
��
�ʴ�Ϊ��
��5��EΪ ��E��ͬ���칹��������������
��E��ͬ���칹��������������
�ٸ�ͬ���칹��ı��������ڵ�����̼ԭ���϶�����ȡ������
�ڸ�ͬ���칹����һ���������ܷ���������Ӧ��ˮ�ⷴӦ������FeCl3��Һ����ɫ��˵������ȩ���������ͷ��ǻ�������������ͬ���칹��ṹ��ʽΪ��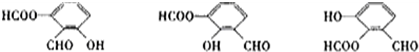 ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
�� ��
��
��6��I�ṹ��ʽΪ ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
��
���� ���⿼���л����ƶϣ�Ϊ��Ƶ���㣬����PETG�ṹ��ʽ��������Ϣ�����ƶϣ����ؿ���ѧ�������ƶϼ�֪ʶǨ���������ѵ��ǣ�5����ͬ���칹��ṹ��ʽȷ������Ŀ�Ѷ��еȣ�

| A�� | ��������Һ | B�� | ����ͭ��Һ | C�� | ��������Һ | D�� | ����������Һ |
| A�� | NaOH��NaCl��HCl��Na2SO4 | B�� | NaCl��Na2SO4��NaOH��HCl | ||
| C�� | NaCl��NaOH��Na2SO4��HCl | D�� | Na2SO4��NaOH��NaCl��HCl |
| A�� | ��Һ�д��ڳ����ܽ�ƽ�⣺AgCl ��s��?Ag+��aq��+Cl-��aq�� | |
| B�� | ʵ�����֤��NH3���Ag+������Cl-ǿ | |
| C�� | ��������Ϣ����֪����Ũ��������ɵij���ΪAgCl | |
| D�� | ʵ�����ʵ���ҿ��ð�ˮϴ��������Ӧ����Թ� |
| A�� | pH��ͬ�Ģ�CH3COONa����NaHCO3����NaClO������Һ��c��Na+�����٣��ڣ��� | |
| B�� | ����AgCl��AgI���������Һ��c��Ag+����c��Cl-��=c��I-�� | |
| C�� | CO2��ˮ��Һ��c��H+����c��HCO3-��=2c��CO32-�� | |
| D�� | �������ʵ�����NaHC2O4��Na2C2O4����Һ��3c��Na+��=2[c��HC2O4-��+c��C2O42-��+c��H2C2O4��] |
| A�� | �ù���������ữ�ĺ����ҽ���Һ����ȡ�⣺2I-+H2O2+2H+�TI2+2H2O | |
| B�� | ij��Һ�д��ڴ���Fe3+��S2-��Cl-��Na+������NaOH��Һ������Ӧ��Fe3++3OH-=Fe��OH��3�� | |
| C�� | ��AlCl3��Һ��Ͷ�������Na��Na+Al3++2H2O=Na++AlO2-+2H2�� | |
| D�� | ��Na2O2����Ͷ��H218O�У�2H218O+2Na2O2=4OH-+4Na++18O2�� |
 CCTV-1�������̸����Ŀ�������������ж��¼��������ٴγ�Ϊ���ڹ�ע�Ľ��㣮��������Ϣ��ͼ��ʾ�����С�6s26p1����ʾ��ԭ����6�����Ӳ㣬�������3�����ӣ������йؿ�Ƭ��Ϣ�������ȷ���ǣ�������
CCTV-1�������̸����Ŀ�������������ж��¼��������ٴγ�Ϊ���ڹ�ע�Ľ��㣮��������Ϣ��ͼ��ʾ�����С�6s26p1����ʾ��ԭ����6�����Ӳ㣬�������3�����ӣ������йؿ�Ƭ��Ϣ�������ȷ���ǣ�������| A�� | ���Ԫ�ط���ΪTl | B�� | ��ԭ�ӵ�������=204-81=123 | ||
| C�� | ��λ�ڵ������ڢ�A�� | D�� | ��Ľ����Ա����Ľ�����ǿ |
| A�� | ���ᡢ���ƫ�����ƺ������Ʒֱ������ᡢ��κ������� | |
| B�� | �Ҵ����������ƺͶ�������ֱ����ڷǵ���ʡ�ǿ����ʺ�������� | |
| C�� | Na��Al��Cuͨ���ֱ��õ�ⷨ���ȷֽⷨ���û���ұ���õ� | |
| D�� | ��Ȼ����������ˮú���ֱ����ڻ�ʯ��Դ����������Դ�Ͷ�����Դ |
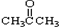 ��CH3CH2Br ��
��CH3CH2Br ��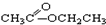 ��
��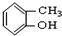 ��
��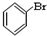 ��
�� ��
��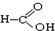 ��
��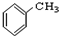 ��
��