��Ŀ����
���Ȼ�������������ɫҺ�壬�ڿ����м���ˮ�⣬�۵�-36�棬�е�114�棬���������۵�Ϊ231�档װ��A�з�Ũ����B�з�MnO2�����������������������ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ������˷�Ӧ���̷ų��������ȣ�����ش����и����⡣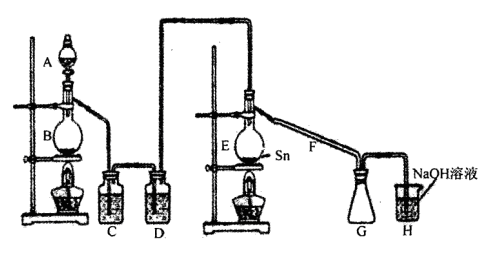
��1������ͼ�����巢����β������װ�ò������ƣ���������Ľ����_______________________________��
���øĽ������ȷװ�ý���ʵ�飬��ش��������⣺
��2��H�з�Ӧ�����ӷ���ʽ��_________________________________________________��
E�з�Ӧ�Ļ�ѧ����ʽ��________________________________________________��
��3��C��D�е��Լ��ֱ���_______________��____________________��
��4������A��B�����Ʒֱ���_____________��____________��F��������_____________��
��5��ʵ��ʱӦ�ȵ�ȼ_________���ƾ��ƣ������¶�Ӧ����________ �棬��________������ֹͣ���ȡ�
��6����֪���Ȼ�����ˮǿ��ˮ�⣬����֮һ�ǹ�̬������������ô���Ȼ���ˮ��Ļ�ѧ����ʽΪ________________________________________________________________��
��7���������ȡ�����Ȼ���������¶�ڿ����У�Ԥ�ڿɿ�����������___________________________��
��
��1���õ��ܽ�A���Ͽں�B��������A���ɺ�ѹ��Һ©����1�֣���G��H֮�����Ӹ���װ�ã�1�֣�
��2��Cl2��H2O=Cl�D��ClO�D��H2O��2�֣�Sn��2Cl2 SnCl4��2�֣�
SnCl4��2�֣�
��3������ʳ��ˮ����ˮ����1�֣�Ũ���ᣨ1�֣�
��4����Һ©����1�֣�������ƿ��1�֣�������������2�֣�
��5��E��1�֣�231�棨1�֣�Sn���ۻ���1�֣�
��6��SnCl4��2H2O=SnO2��4HCl��2�֣�
��7�����ְ�ɫ������1�֣�
���������������1������װ�����������壬ѹǿ����Һ©���е������˳���ӵ�Բ����ƿ�У�Ӧ�õ��ܽ�A���Ͽ���B��������ƽ���Һ©����Բ����ƿ�е�ѹǿ�����Ȼ�������ˮ�⣬GΪ�ռ����Ȼ���װ�ã�Ӧ��G��H֮�����Ӹ���װ�÷�ֹH�е�ˮ��������Gװ�ã���Ϊ���õ��ܽ�A���Ͽ���B��������G��H֮�����Ӹ���װ�ã�
��2��HΪ����Ϊ��Ӧ���������������������Ʒ�Ӧ�����Ȼ��ơ��������ơ�ˮ����Ӧ���ӷ���ʽΪ��Cl2+2OH��=Cl��+ClO��+H2O����װ��ͼ��֪��E��Ϊ�������������������Ӧ������ˮ���Ȼ�������Ӧ����ʽΪ��Sn+2Cl2 SnCl4����Ϊ��Cl2+2OH��=Cl��+ClO��+H2O��Sn+2Cl2
SnCl4������Cl2+2OH��=Cl��+ClO��+H2O��Sn+2Cl2 SnCl4��
SnCl4��
��3�����Ȼ�������ˮ�⣬����װ��E������Ӧ���������װ��C������Ϊ���ջӷ�����HCl���ñ���ʳ��ˮ����HCl��װ��D������Ϊ����ˮ�������������壬��Ũ�������ո����Ϊ������ʳ��ˮ��Ũ���
��4������AΪ��Һ©����BΪ������ƿ��F���������������Ȼ�����������Ϊ����Һ©����������ƿ��������������
��5�����ڵĽ����������������ֱ��������ȡ��ˮ���Ȼ�������Ӧ�����E�ƾ��ƣ������¶�Ӧ���ڽ��������۵㣬�����ڻ���ֹͣ���ȣ���Ϊ��E��231�����ڻ���
��6�����Ȼ�����ˮǿ��ˮ�⣬��ˮ��ԭ����֪��Ӧ����Sn(OH)4��HCl������֮һ�ǹ�̬����������˵��Sn(OH)4�ֽ�����SnO2��H2O�������Ȼ���ˮ������SnO2��HCl����Ӧ����ʽΪ��SnCl4+2H2O=SnO2+4HCl����Ϊ��SnCl4+2H2O=SnO2+4HCl��
��7�����Ȼ�����ˮǿ��ˮ������SnO2��HCl��SnO2�ǹ��������HCl��Ͽ����е�ˮ���������ְ�ɫ��������Ϊ�����ְ�ɫ������
���㣺������ʵ�����Ʒ�

 ����С״Ԫ��������������ϵ�д�
����С״Ԫ��������������ϵ�д�ij��ѧС��������װ�ó�ȡ�ռ����������������о������ʡ�������������⡣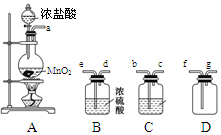
��1��װ��A�з�����Ӧ�����ӷ���ʽΪ_______________________________��
��2��������������������ӿڵ�����˳��Ϊa��___________________��g��
��3��װ��B��Ũ�����������____________________________________________________________��װ��C���Լ������___________________________________��
��4��ijͬѧ��Ϊ��������ȱ��β������װ�ã���������ķ����л�����װ�ò�ע���Լ���
| |
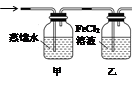
��5��װ��ȡ������ͨ����ͼ��ʾװ���У���װ����Һ�о��������Եĺ���������_______�����֤����װ����FeCl2��Һ��Cl2�����˷�Ӧ����ֻ�ش���Ҫ���Լ�������___________________________��
ClO2��Ϊ�����������������ж��������������й㷺��Ӧ��ǰ����ijͬѧ����ͼ��ʾ��װ���Ʊ�ClO2���壬��Ӧԭ��Ϊ���Ͳ�����Һ��KClO3��ĩ��60��ʱ��Ӧ�Ƶ�ClO2���¶ȹ�����Ͷ���Ӱ������Ч�ʣ�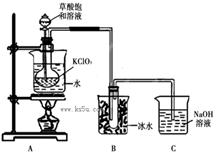
��֪��Ϣ����ClO2��һ�ֻ���ɫ�д̼�����ζ�����壬�۵�-59�棬�е�11.0�档��Ӧ���������ơ�
�ڲ���������ǿ�ڴ���Ķ�Ԫ���ᣬ��Ӧ�ĸ��Σ�CaC2O4�������ڴ��ᣬ������ǿ�ᣬ������һ�ֻ�ԭ�Խ�ǿ�����ʡ�
��1���Ʊ�ClO2�Ļ�ѧ����ʽ��2KClO3+H2C2O4= 2KHCO3+2ClO2��������˵����ȷ����
| A��KClO3�ڷ�Ӧ�еõ����� |
| B��ClO2���������� |
| C��H2C2O4�ڷ�Ӧ��ʧȥ���� |
| D��1mol KClO3�μӷ�Ӧ��2mol����ת�� |
����A���ձ�ȥ��������ƿֱ�Ӽ����Ƿ���ԣ�Ϊʲô��_________________________��
��3��Cװ������β������Һ�к���NaOH��Na2CO3�����ʣ���ͬѧ��ΪC�л����ܺ���NaClO2��NaClO3���Է������ܵ�ԭ��д���йػ�ѧ����ʽ________ ��
��4��ʵ���һ���ͨ�����·�Ӧ�Ƶ�ClO2��
KClO3�� H2C2O4�� H2SO4= ClO2���� K2SO4�� CO2��+ H2O.
��ƽ��������ʽ��0.5 mol KClO3�μӷ�Ӧ�� ������ת�ơ�
����������һ�ִ�����Ⱦ��о���NO2��SO2��CO�ȴ�����Ⱦ����Ĵ�������Ҫ���壬ij��ѧʵ�鰮��С����̽��SO2�����ʣ�������·�����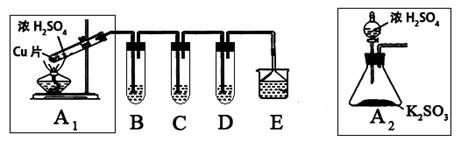
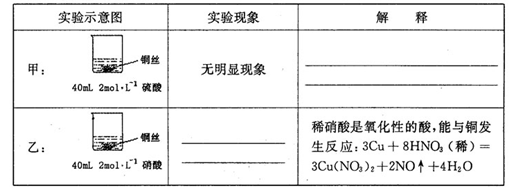
��1��B��C��D�ֱ����ڼ���SO2�Ļ�ԭ�ԡ������Ժ�Ư���ԡ�����B��C�ֱ�Ϊ��ˮ�������ˮ��Һ����D����ʢ�Լ�Ϊ_________��B�з�Ӧ�����ӷ���ʽΪ��_________________��
��2��Ϊ��ʵ����ɫʵ���Ŀ�꣬ijͬѧ���������������ͼA2����ȡװ��������A1װ�ã���A1װ����ȣ�A2װ�õ��ŵ��ǣ�________________________________����дһ�㼴�ɣ���
��3��E���ð�ˮ����β���е�SO2��������Һ���п��ܺ���OH����SO32����SO42����HSO3���������ӡ���֪����������һ��������ˮ��SO2Ҳ������ˮ�������������Լ�Ϊ��С�ձ����Թܡ�����������ͷ�ιܡ�����װ�ú���ֽ��2mol/L���ᡢ1mol/L BaCl2��Һ��1mol/L Ba(OH)2��Һ��Ʒ����Һ������ˮ��
�����ʵ��֤��������Һ���д���SO32����HSO3��������±���ʵ�������Ԥ������ͽ��ۣ�
| ʵ����� | Ԥ����������� |
| ����1��ȡ����������Һ����С�ձ��У��ý�ͷ�ι�ȡ1mol/L BaCl2��Һ��С�ձ��μ�ֱ�������� | �����ְ�ɫ���ǣ�����Һ�д���SO32���� SO42���� |
| ����2����С�ձ��е���Һ���ˡ�ϴ�ӣ���������ˮ�Ѹ�����ֽ�ϵĹ��������һС�ձ��У�����µĹ��� ___________________________________________________ | _________________________ ______________________________________________ |
| ����3��_______ _______________________ ___________________________________________________ | _________________________ |
��ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���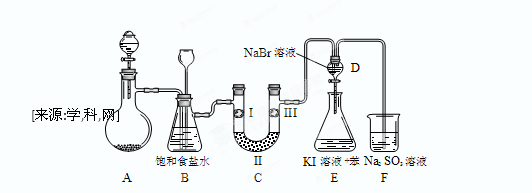
��1���Ʊ�����ѡ�õ�ҩƷΪ��Ư�۾����塾��Ҫ�ɷ�ΪCa��ClO��2����Ũ���ᣬ��صĻ�ѧ��Ӧ����ʽΪ�� ��
��2��װ��B�б���ʳ��ˮ�������� ��ͬʱװ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������д����������ʱB�е����� ��
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C��I��II��III���η��� ��
| | a | b | c | d |
| I | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| II | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| III | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��4�����װ��D��E��Ŀ���DZȽ��ȡ��塢��ķǽ����ԡ�����D�л���ͨ����������ʱ�����Կ�����ɫ��Һ��Ϊ����ɫ��˵���ȵķǽ����Դ����塣��������װ��D��������Һ����װ��E�У����۲쵽�������� �������� ����ܡ����ܡ���˵����ķǽ�����ǿ�ڵ⣬ԭ���� ��
X��Y��Z���ֲ�ͬ��������ͼ��ʾ��ת����ϵ����X�������� (����)��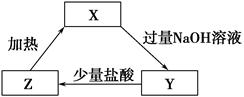
| A��Al2O3 | B��SiO2 | C��CO2 | D��NH4Cl |
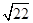 =0.7��
=0.7��