��Ŀ����
ijͬѧ��ѧϰ����������ʱ������������ͭ�ķ�Ӧ��������о�����������и��⡣
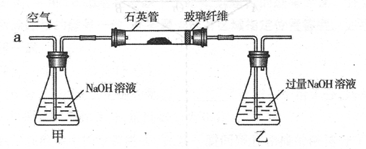
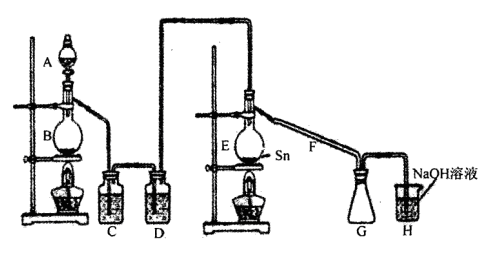
��1���ڼס��������ձ��У��ֱ�װ��40mLŨ�Ⱦ�Ϊ2mol��L��1��ϡ�����ϡ���ᣬ�������и����� 4g��״ͭ˿���۲��������������ʵ�鱨�棺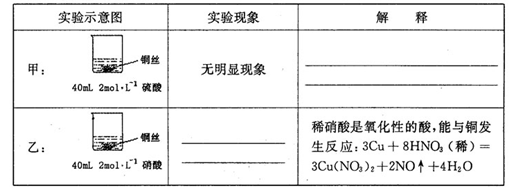
��2����ַ�Ӧ���ס����ձ���ϣ���ʹ֮��ַ�Ӧ������������Һ����Ϊ____ ��ʣ�����������Ϊ g
��3��������������Һ���V��V>40mL���ɱ䣬�������ݲ��䣬��
�ٵ��ס����ձ���ϳ�ַ�Ӧ����Һ��ֻ��һ������ʱ��V=____ mL����Ҫ����Һ�е�Cu2+������ȫ��Ӧ��NaOHʹ��Һ��pH����Ϊ____ ����֪KsP[Cu��OH��2]=2.2��l0��20,1g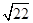 =0.7��
=0.7��
���ܷ�ͨ��������Һ����ĸı䣬ʹͭ˿�ڼס����ձ���ϳ�ַ�Ӧ����ȫ�ܽ�? ��д����������________ ��
26����1���ף�ϡ����ֻ�ܱ�����������ԣ���ͭ���ڽ����˳�����֮���ܽ����û�����
��1�֣��� �ң�ͭ˿���ܽ⣬ͭ˿���������ݲ�������Һ��ɫ������1�֣���
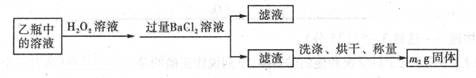
��2��CuSO4��Cu(NO3)2��2�֣���2.24��2�֣���3����60��2�֣� 6.7��2�֣��ڷ�1�֣�����3�֣�
���������������1���ף�����ϡ����ֻ�ܱ�����������ԣ���ͭ���ڽ����˳�����֮�����Բ��ܽ����û��������ң������������������ᣬ����������ͭ�����ʵ��������ͭ˿���ܽ⣬ͭ˿���������ݲ�������Һ��ɫ������
��2���������������ʵ�����0.04L��2mol/L��0.08mol������NO3�������ʵ�����0.08mol�������ӵ����ʵ�����0.24mol�����ݷ���ʽ3Cu+8H++2NO3��=3Cu2++2NO��+4H2O��֪�����Ӳ��㣬NO3���������������ܽ�ͭ��������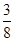 ��0.24mol��64g/mol��5.76g��8g������ͭ������ʣ��ͭ��������8g��5.76g��2.24g��1������Һ������������Һ����ΪCuSO4��Cu(NO3)2��
��0.24mol��64g/mol��5.76g��8g������ͭ������ʣ��ͭ��������8g��5.76g��2.24g��1������Һ������������Һ����ΪCuSO4��Cu(NO3)2��
��3��������Һ������ֻ��һ�֣��������Ӧ��������ͭ���������ӷ���ʽ3Cu��8H+��2NO3��=3Cu2+��2NO����4H2O��֪����Ҫ�����ӵ����ʵ�����0.08mol��4��0.032mol�����������ṩ����������0.032mol��0.08mol��0.24mol��������������ʵ�����0.24mol��2��0.12mol����������Һ�������0.12mol��2mol/L��0.06L��60ml������Һ��ͭ����Ũ��С�ڻ����10��5mol/Lʱ���Կ���ͭ������ȫ������������ܶȻ�������֪����ʱ��Һ��c(OH��)��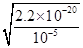 ��
��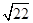 ��10��8mol/L����c(H+)��
��10��8mol/L����c(H+)��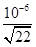 �����pH��6��1g
�����pH��6��1g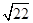 ��6.7��
��6.7��
�����ձ����е�NO3����40mL��10-3L/mL��2mol/L��0.08mol�������ӷ���ʽ3Cu��8H+��2NO3��=3Cu2+��2NO����4H2O������������Cu������Ϊ0.08mol��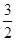 ��64g/mol��7.68g��С�����ձ���ͭ˿����������������ͭ�ܽ��ꡣ
��64g/mol��7.68g��С�����ձ���ͭ˿����������������ͭ�ܽ��ꡣ
���㣺����ͭ�����ᡢ��������ʣ�ͭ�����ᷴӦ�ļ��㣻�ܶȻ�������Ӧ�õ�

��ʵ���������ʼ�ֱ��ת����Ԫ����
| A���� | B���� | C��ͭ | D���� |
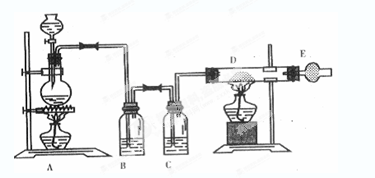

 CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
CO2��+2SO2��+2H2O������ŨH2 S04����������� �������������ԭ������������0��2mol̼����ȫ��Ӧ��������H2S04�������� g������²���SO2�����Ϊ______________L��
 [
[ 2Fe2O3 + 8SO2
2Fe2O3 + 8SO2