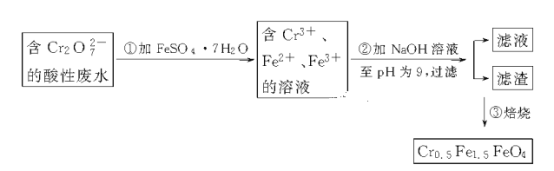
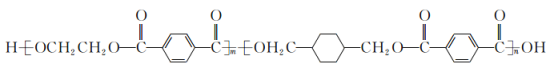��Ŀ����
����Ŀ����ͼ��ʾ��װ�â�Ϊ���͵�أ��ŵ�ʱB�缫�ķ�ӦʽΪNaBr3+2Na++2e-��3NaBr��װ�â�ΪǦ���ء����ȱպ�K1��K2����Ǧ���س������Ͽ�K1���պ�K2������˵����ȷ����

A.װ�â�ŵ�ʱ��A�缫��ӦʽΪ2Na2S2-2e-��Na2S4+2Na+
B.�պ�K1��K2ʱ��ÿ��0.1molNa+ͨ�����ӽ���Ĥ��װ�â���Һ����0.1mol����ת��
C.�Ͽ�K1���պ�K2ʱ��b�缫�ĵ缫��ӦʽΪPbO2+2e-+SO42-+4H+��PbSO4+2H2O
D.�Ͽ�K1���պ�K2ʱ��װ�â���SO42-��a�缫Ǩ��
���𰸡�A
��������
�ɷŵ�ʱ�缫B�Ϸ�����ӦNaBr3��2Na����2e��=3NaBr��֪���缫BΪ�������缫AΪ������A�缫�ĵ缫��ӦʽΪ��2Na2S2��2e��=2Na����Na2S4��
A��װ�â�ŵ�ʱ���ܷ�ӦʽΪ��2Na2S2��NaBr3=Na2S4��3NaBr��ѡ��A��ȷ��
B�����պ�K1���Ͽ�K2��ÿ��0.1 mol Na��ͨ�����ӽ���Ĥ����ת��0.1 mol e��������Һ���ܴ��ݵ��ӣ�ѡ��B����
C���Ͽ�K1���պ�K2ʱ��Ǧ���طŵ磬b�缫������������ʧ���ӵ�������Ӧ��ѡ��C����
D��ԭ��طŵ�ʱ���������ƶ�����SO42-��b�缫�ƶ���ѡ��D����
��ѡA��

 �����������һ��һ��ϵ�д�
�����������һ��һ��ϵ�д�����Ŀ����һ�ܱ������г���A��B��C�������壬����һ���¶ȣ���t1��t4ʱ�̲�ø����ʵ�Ũ�����±���
�ⶨʱ��/s | t1 | t2 | t3 | t4 |
c(A)/(mol��L-1) | 6 | 3 | 2 | 2 |
c(B)/(mol��L-1) | 5 | 3.5 | 3 | 3 |
c(C)/(mol��L-1) | 1 | 2.5 | 3 | 3 |
�ݴ��ж����н�����ȷ���ǣ� ��
A.��t3ʱ�̷�Ӧ�Ѿ�ֹͣ
B.t1��t4ʱ�̣�A��ת���ʱ�B��ת���ʵ�
C.�������з����ķ�ӦΪ2A��B![]() 2C
2C
D.����ѹǿ����ƽ��ʱ��������ƽ����Է�����������