��Ŀ����
2��������������������մ���Һ���������Ժͽ�ǿ�Ļ�ԭ�ԣ�����֯��Ư������ȼ������������еĻ�ԭ������������ƣ�Na2S2O3�������������ƺ����ͨ�����Ϸ�Ӧ�Ƶã�װ����ͼ1��ʾ��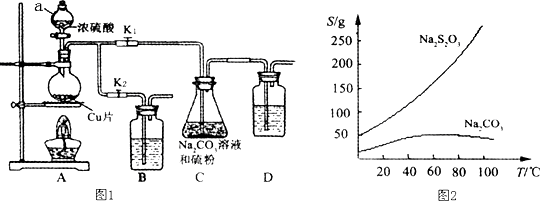
��֪��Na2S2O3��������Һ�в����ȶ����ڣ��й����ʵ��ܽ��������ͼ2��ʾ��
��1��Na2S2O3•5H2O���Ʊ���
����1��ֻ���Aװ�������ԵIJ����ǹر�K1��K2�����ϲ������������Һ©���м�ˮ��һ��ʱ���©����Һ�治���½���˵�����������ã�
����2������ҩƷ����K1���ر�K2�����ȣ�װ��B��D�е�ҩƷ��ѡ�����������е�ABCD�����ţ�
A��NaOH��Һ B����ˮ
C������KMnO4��Һ D������NaHCO3��Һ
����3��C�л��Һ��������������Ӧһ��ʱ�����۵������٣�
����4������C�еĻ��Һ������Һ��������Ũ�������ȹ��ˣ��õ�Na2CO3���ѧʽ�����ٽ���Һ��ȴ�ᾧ����������ƣ������ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��2���ƵõĴ־��������������������ʣ�Ϊ�˲ⶨ�ֲ�Ʒ��Na2S2O3•5H2O�ĺ�����һ�������������������KMnO4��Һ�ζ��ķ������ٶ��ֲ�Ʒ������������KMnO4��Һ����Ӧ����
��ȡ1.92g�Ĵ���Ʒ����ˮ����0.40mol/L KMnO4��Һ���������������ữ���ζ�������Һ��S2O32-ȫ��������ΪSO42-ʱ������KMnO4��Һ���20.00mL���Իش�
��KMnO4��Һ������ʽ �����ʽ����ʽ�����ζ����У�
�ڵζ��յ�ʱ����ɫ�仯����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
�۲�Ʒ��Na2S2O3•5H2O����������Ϊ64.4%��
���� ��1������1����������ѹǿ������ɷ������ʣ�����Aװ�������ԣ�
����2��װ��B��D�������ǽ���β����������ֹβ���ж���������Ⱦ������
����3��C�л��Һ��������������Ӧһ��ʱ�����۵������٣�
����4������Һ�л�þ��壬��Ҫ����Ũ�������ȹ��ˣ��õ�Na2CO3���ٽ���Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
��2�������Ը��������Һ����ǿ�����ԣ��ܹ�������ʽ�ζ��ܵ��ܣ�ֻ��ʹ����ʽ�ζ��ܣ�
�ڷ�Ӧ����ǰ��ҺΪ��ɫ����Ӧ��������Һ�и���������ӹ�������Һ��Ϊdz��ɫ��
�۸���n=cV�����1.92g��Ʒ���ĵĸ�����ص����ʵ��������ݸ��ݷ�Ӧ�������Ʒ�к���Na2S2O3•5H2O�����ʵ������ٸ������������ı���ʽ�������Ʒ��Na2S2O3•5H2O������������
��� �⣺��1������1����������ѹǿ������ɷ������ʣ�����Aװ�������ԣ��������Ϊ���ر�K1��K2�����ϲ������������Һ©���м�ˮ��һ��ʱ���©����Һ�治���½���˵�����������ã�
����2��װ��B��D�������ǽ���β����������ֹβ���ж���������Ⱦ����������������л�ԭ�ԣ����������Ը��������Һ������ˮ�������գ�����������������������Һ��̼��������Һ��Ӧ�����գ���ѡ��ABCD��
����3��C�л��Һ��������������Ӧһ��ʱ�����۵������٣�
����4������Һ�л�þ��壬��Ҫ����Ũ�������ȹ��ˣ��õ�Na2CO3���ٽ���Һ��ȴ�ᾧ�����ˡ�ϴ�ӡ���ɣ��õ���Ʒ��
�ʴ�Ϊ���ر�K1��K2�����ϲ������������Һ©���м�ˮ��һ��ʱ���©����Һ�治���½���˵�����������ã�ABCD��Na2CO3����ȴ�ᾧ��
��2�����������Ը��������Һ�ܹ�������ʽ�ζ��ܵ��ܣ����Եζ����������Ը��������Һ�����ü�ʽ�ζ���ʢ�ţ�Ӧ������ʽ�ζ��ܣ�
�ʴ�Ϊ����ʽ��
�����ݱ궨��ԭ����֪����Ӧ����ǰ��ҺΪ��ɫ����Ӧ��������Һ�и���������ӹ�������Һ��Ϊdz��ɫ�����Եζ��յ������Ϊ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
�ʴ�Ϊ����Һ����ɫ��Ϊdz��ɫ��������ڲ���ɫ��
��20mL 0.40mol/L KMnO4��Һ�к��и�����ص����ʵ���Ϊn��KMnO4��=0.40mol/L��0.02L=0.008mol��
���ݷ�Ӧ5S2O32-+8MnO4-+14H+�T8Mn2++10SO42-+7H2O��֪��1.28g�Ĵ���Ʒ����Na2S2O3•5H2O�����ʵ���Ϊ��n��Na2S2O3•5H2O��=n��S2O32-��=$\frac{5}{8}$��n��KMnO4��=0.005mol��
��Ʒ��Na2S2O3•5H2O����������Ϊ��$\frac{248g/mol��0.005mol}{1.92g}$��100%=64.4%��
�ʴ�Ϊ��64.4%��
���� ����ͨ��Na2S2O3•5H2O���Ʊ�����������������ʵ�鷽����Ʒ�����Ϊ�߿��ĸ�Ƶ�⣬�Ѷ��еȣ���ȷ���������Ϣ��ȷ�Ʊ�ԭ��Ϊ��������Ĺؼ��������ֿ�����ѧ���ķ�����������������ѧʵ����������һ�������ϸߵ���Ŀ��

| A�� | �����۳�ʢ��Ũ����--ǿ������ | |
| B�� | ����Ũ������--���ȶ��� | |
| C�� | Ũ������ʹ���DZ�ڲ�����--��ˮ�� | |
| D�� | ��ϡ����ϴ������������Ӧ���Թ�--ǿ�����Ժ����� |
| A�� | ͬʱ���� | B�� | ͬʱ��С | C�� | v1����v2��С | D�� | v1��С��v2���� |
| A�� | Al������������Al2O3��Fe��������Ҳ����Fe2O3 | |
| B�� | ��VA���⻯����۷е�˳����NH3��AsH3��PH3����� VIA���⻯����۷е�˳��Ҳ��H2O��H2Se��H2S | |
| C�� | ��ҵ���õ�����ڵ��Ȼ�þ�Ʊ�þ���ʣ���ҵ��Ҳ���õ�����ڵ��Ȼ����Ʊ������� | |
| D�� | BaCl2��Һ��ͨ��SO2������������Ba��NO3��2��Һ��ͨ��SO2Ҳ�������� |
| A�� | NH3���������������������������� | |
| B�� | Mg��Fe�Ƚ�����һ����������ˮ��Ӧ������H2�Ͷ�Ӧ���������� | |
| C�� | Fe�������е�ȼ�ղ���������ƺ�ɫͿ�� | |
| D�� | Mg��OH��2�ֽ����������ɸ��۵���壬������ȼ�� |
| A�� | �ס����ж��������� | B�� | ���������������м���� | ||
| C�� | ��������������������� | D�� | ����������������� |
��1����PM2.5����������ˮ�����Ƴɴ���������
����ø���������ˮ���������ӵĻ�ѧ��ּ���ƽ��Ũ�����±���
| ���� | K+ | Na+ | NH4+ | SO42- | NO3- | Cl- |
| Ũ��/mol•L-1 | 4��10-6 | 6��10-6 | 2��10-5 | 4��10-5 | 3��10-5 | 2��10-5 |
��2��Ϊ����SO2���ŷţ�����ȡ�Ĵ�ʩ�У�
�ٽ�úת��Ϊ�������ȼ�ϣ�
��֪��H2��g��+$\frac{1}{2}$O2��g���TH2O��g����H=-241.8kJ•mol-1
C��s��+$\frac{1}{2}$O2��g���TCO��g����H=-110.5kJ•mol-1
д����̿��ˮ������Ӧ���Ȼ�ѧ����ʽ��C��s��+H2O��g���TCO��g��+H2��g������H=+13l.30kJ•mol-1��
��ϴ�Ӻ�SO2���������������ʿ���ϴ�Ӽ�����ab��
a��Ca��OH��2 b��Na2CO3
c��CaCl2 d��NaHSO3
��Ŀǰ��������β��ϵͳ��װ�ô�ת�����ɼ���CO��NO����Ⱦ���仯ѧ��Ӧ����ʽΪ2CO+2NO $\frac{\underline{\;����\;}}{\;}$2CO2+N2��