��Ŀ����
18�� ̼��̼�Ļ������������������е�Ӧ�÷dz��㷺�����ᳫ���������ѳɳ����Ľ��죬����̼�������ֻ�����룬����һ��ֵ���ڴ����µ����ʽ�������û�ѧ��Ӧԭ�������֪ʶ�о�̼���仯��������ʣ�
̼��̼�Ļ������������������е�Ӧ�÷dz��㷺�����ᳫ���������ѳɳ����Ľ��죬����̼�������ֻ�����룬����һ��ֵ���ڴ����µ����ʽ�������û�ѧ��Ӧԭ�������֪ʶ�о�̼���仯��������ʣ���1�����������ҹ���������̼���о�ȡ���ش��չ���õ绡���ϳɵ�̼�����г����д���̼�����������ʣ�������̼���������������������ᴿ���䷴Ӧ��ѧ����ʽΪ��
3C+2K2Cr2O7+8H2SO4=3CO2��+2K2SO4+2Cr2��SO4��3+8H2O
����ɲ���ƽ������ѧ����ʽ��������������K2Cr2O7������������CO2
��2����ҵ��һ���ں����ܱ������в������з�Ӧ�ϳɼ״���CO��g��+2H2��g��?CH3OH��g����H
�±����������Ǹ÷�Ӧ�ڲ�ͬ�¶��µĻ�ѧƽ�ⳣ����������
| �� �� | 250�� | 300�� | 350�� |
| �� | 2.041 | 0.270 | 0.012 |
��ij�¶��£���2mol CO��6mol H2����2L���ܱ������У���ַ�Ӧ 10min�ﵽƽ��ʱ���c��CO��=0.2mol/L�����ʱ���¶�Ϊ250�森
���������������еĻ�����������¶���CO��H2�� CH30H��Ũ����ʱ��仯�����ߣ��������ʵ��ı�ע��
��3����ҵ��Ҳ������CO2��H2��Ӧ�Ƶü״�����2��105Pa��300��������£�����440g CO2��H2ǡ����ȫ��Ӧ���ɼ״���ˮ���ų�495kJ����������д���÷�Ӧ���Ȼ�ѧ����ʽCO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ•mol-1��
��4����CH3OHΪȼ�ϣ��� KOH ��Һ���������Һ�����Ƴ�CH3OHȼ�ϵ�أ������CH3OH�ĵ缫Ϊ����������O2�缫�ķ�ӦʽΪO2+4e-+2H2O=4OH-��
���� ��1����Ӧ��CԪ�ػ��ϼ���0������Ϊ+4�����ϼ����߹�4�ۣ�CrԪ�ػ��ϼ���+�۽���Ϊ+3�����ϼ��ܽ���6�ۣ����ϼ�������С������Ϊ12����C��ϵ��Ϊ3��K2Cr2O7ϵ��Ϊ2���ٸ���ԭ���غ���ƽ����֪ȱ��ΪH2SO4��
����Ԫ�ػ��ϼ۽��͵ķ�Ӧ��Ϊ������������Ԫ�ػ��ϼ����߷�Ӧ��Ϊ��ԭ������ԭ������������Ӧ�����������
��2�����ɱ������ݿ�֪�����¶�����ƽ�ⳣ����С��˵�������¶�ƽ�����淴Ӧ�����ƶ�������ӦΪ���ȷ�Ӧ��
�ڼ���ƽ��ʱ����ֵ�Ũ�ȣ�����K=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$����ƽ�ⳣ��������ȷ���¶ȣ�
���淴Ӧ���У���Ӧ��Ũ�Ƚ��͡�������Ũ��������ͼ��ע�����ʵ���ʼŨ�ȡ�ƽ��Ũ���Լ���Ӧ����ƽ���ʱ�䣻
��3��������Ӧ��CO2+3H2=CH3OH+H2O������1mol������̼��Ӧ�ų���������ע�����ʵľۼ�״̬�뷴Ӧ����д�Ȼ�ѧ����ʽ��
��4����CH3OHΪȼ�ϣ� KOH ��Һ���������Һ�����Ƴ�CH3OHȼ�ϵ�أ��״�����������Ӧ���ڸ������룬����������ԭ��Ӧ����õ��ӣ������������������������ӣ�
��� �⣺��1����Ӧ��CԪ�ػ��ϼ���0������Ϊ+4�����ϼ����߹�4�ۣ�CrԪ�ػ��ϼ���+�۽���Ϊ+3�����ϼ��ܽ���6�ۣ����ϼ�������С������Ϊ12����C��ϵ��Ϊ3��K2Cr2O7ϵ��Ϊ2���ٸ���ԭ���غ���ƽ����֪ȱ��ΪH2SO4����ƽ��ʽΪ��3C+2K2Cr2O7+8 H2SO4=2CO2��+2K2SO4+2Cr2��SO4��3+8H2O��������������K2Cr2O7������������CO2��
�ʴ�Ϊ��3��2��8 H2SO4=3��2��2��8��K2Cr2O7��CO2��
����Ԫ�ػ��ϼ۽��͵ķ�Ӧ��Ϊ������������Ԫ�ػ��ϼ����߷�Ӧ��Ϊ��ԭ������ԭ������������Ӧ�����������
��2�����ɱ������ݿ�֪�����¶�����ƽ�ⳣ����С��˵�������¶�ƽ�����淴Ӧ�����ƶ����������¶�ƽ�������ȷ�Ӧ�ƶ���������ӦΪ���ȷ�Ӧ������H��0
�ʴ�Ϊ������
��ij�¶��£���2mol CO��6mol H2����2L���ܱ������У���ַ�Ӧ 10min�ﵽƽ��ʱ���c��CO��=0.2mol/L����
CO��g��+2H2��g��?CH3OH��g��
��ʼ����mol/L����1 3 0
�仯����mol/L����0.8 1.6 0.8
ƽ������mol/L����0.2 1.4 0.8
ƽ�ⳣ��K=$\frac{c��C{H}_{3}OH��}{c��CO����{c}^{2}��{H}_{2}��}$=$\frac{0.8}{0.2��1��{4}^{2}}$=2.401�����¶�Ϊ250�棬
�ʴ�Ϊ��250�棻
���淴Ӧ���У���Ӧ��Ũ�Ƚ��͡�������Ũ������������¶���CO��H2�� CH30H��Ũ����ʱ��仯������Ϊ��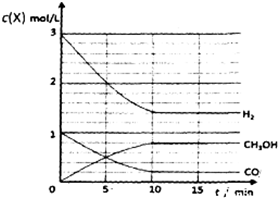 ��
��
�ʴ�Ϊ�� ��
��
��3��������Ӧ��CO2+3H2=CH3OH+H2O��1mol������̼��Ӧ�ų�������Ϊ495kJ��$\frac{1mol��44g/mol}{440g}$=49.5kJ���ʷ�Ӧ�Ȼ�ѧ����ʽΪ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ•mol-1��
�ʴ�Ϊ��CO2��g��+3H2��g��=CH3OH��g��+H2O��g����H=-49.5KJ•mol-1��
��4����CH3OHΪȼ�ϣ� KOH ��Һ���������Һ�����Ƴ�CH3OHȼ�ϵ�أ��״�����������Ӧ���ڸ������룬����������ԭ��Ӧ����õ��ӣ������������������������ӣ��缫��ӦʽΪ��O2+4e-+2H2O=4OH-��
�ʴ�Ϊ������O2+4e-+2H2O=4OH-��
���� ���⿼��ƽ�ⳣ�����㼰Ӱ�����ء�������ԭ��Ӧ��ƽ���Ȼ�ѧ����ʽ��д��ԭ��صȣ���Ŀ�Ƚ��ۺϣ��ϺõĿ���ѧ�����������������Ѷ��еȣ�

��1�����£�N2H4��Ϊȼ�ϣ������������������������߷�Ӧ���ɵ�������̬ˮ��
��֪��N2��g��+2O2��g��=N2O4��g����H1 K1
N2H4��g��+O2��g��=N2��g��+2H2O��g����H2 K2
��2N2H4��g��+N2O4��g��=3N2��g��+4H2O��g����H=2��H2-��H1 ���á�H1����H2��ʾ�����÷�Ӧ�Ļ�ѧƽ�ⳣ��K=$\frac{{{K}_{2}}^{2}}{{K}_{1}}$����K1��K2��ʾ��
��2������β��ת����Ӧ��NO+CO��N2+CO2��δ��ƽ������NO��COת��Ϊ��N2 ��CO2��ʵ�ֳ��ۣ�ÿ����1molN2��ԭ��ʧȥ������Ϊ4��6.02��1023��
��3��������Ⱦ�ﵪ����������û���̿��ԭ��������ij�о�С����ij2L���ܱ������м���һ�����Ļ���̿��NO��������ӦC��s��+2NO��g��?N2��g��+CO2 ��g������T1��ʱ����Ӧ���е���ͬʱ���ø����ʵ����ʵ������£�
| ʱ�䣨min�� ���ʵ�����mol�� | 0 | 10 | 20 | 30 | 40 | 50 |
| NO | 2.00 | 1.16 | 0.80 | 0.80 | 0.96 | 0.96 |
| N2 | 0 | 0.42 | 0.60 | 0.60 | 0.72 | 0.72 |
| CO2 | 0 | 0.42 | 0.60 | 0.60 | 0.72 | 0.72 |
��30min��ֻ�ı�ijһ��������Ӧ���´ﵽƽ�⣬�����ϱ��е������жϸı������������b������ĸ��ţ���
a������һ�����Ļ���̿ b��ͨ��һ������NO c������ʱ������һ�����ĺ��� d��������ʵĴ���
��4����ȼ�ϵ��ʹ�õĵ������Һ��2mol•L-1��KOH��Һ����ط�ӦΪ��4NH3+3O2=2N2+6H2O���ŵ�ʱ���õ�������ĵ缫��ӦʽΪO2+2H2O+4e-=4OH-��
��5��ʵ��������NaOH��Һ����CO2��������ӦΪ2CO2+3NaOH=Na2CO3+NaHCO3+H2O�����û��Һ����������Ũ���ɴ�С��˳��Ϊc��Na+����c��HCO3-����c��CO32-����c��OH-����c��H+����
| �� �� | 2��4��6 �۵�/�� | �е�/�� | �ܶ�/g•cm-3 |
| �� �� | -114 | 78 | 0.789 |
| �� �� | 16.6 | 117.9 | 1.05 |
| �������� | -83.6 | 77.5 | 0.900 |
| ŨH2SO4 | 338 | 1.84 |

ʵ������������������Ҫװ������ͼI��ʾ����Ҫ����Ϊ��
����30mL�Ĵ��Թ��а������1��4��4�ı�������Ũ���ᡢ�Ҵ�������Ļ��Һ��
�ڰ���ͼI����װ�ã�ʹ����������������ͨ��15mL�Թ���ʢ����Na2CO3��Һ������1�η�̪��Һ���Ϸ�2mm��3mm����
��С������Թ��еĻ��Һ��
�ܴ�С�Թ����ռ�Լ4mL����ʱֹͣ���ȣ�����С�Թܲ�������Ȼ���ô���ֲ㣻
�ݷ��������������������
��ͬѧ�ǻش��������⣺
��1��������У�������һ�����Ļ��Һ�IJ������ȼ�������Ҵ���4mL���ٻ�������1mLŨH2SO4���ӱ���
��2��д���÷�Ӧ�Ļ�ѧ����ʽCH3COOH+CH3CH2OH$?_{��}^{Ũ����}$CH3COOC2H5+H2O��ŨH2SO4�������Ǵ�������ˮ����
��3��������У���С������Թ��еĻ��Һ����ԭ����������ᡢ�Ҵ������������е�ӽ��ҽϵͣ������ȣ���Ӧ�����������ʧ��
��4����������۲쵽����������dz��ɫNa2CO3��Һ�ϲ���Լ4cm�����ɫҺ�壬��Na2CO3��Һ���ɫ��dz�������ݣ��ϲ�Һ��䱡��ԭ�����ϲ����Ͳ���Ϊ���ɵ���������������ˮ�����ܶȱ�ˮС��ͬʱ��Ϊ�ӷ�������������̼���Ʒ�Ӧ���ų�CO2���壬���������ݳ��֣�
��5��������У��������������ѡ�õ������Ƿ�Һ©��������Ӧ���Ͽڵ�������Ϊ����������ˮ�ܶ�С��
��6��Ϊ������������IJ��ʣ��ס�����λͬѧ�ֱ��������ͼ��ס��ҵ�װ�ã���ͬѧ����Ӧ�����ȴ�����ñ���Na2CO3��Һ��ȡ��ƿ�в��������Ϊ����װ�ú�����Ϊʲô��
���ң���Ӧ��������������
| A�� | CH3CH2CH2OH | B�� | CH3OCH3 | C�� | CH2=CH-CH3 | D�� | һ�ȱ� |
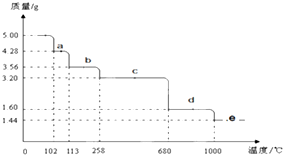 Cu2S�ǻ���ͭ����Ҫԭ��֮һ����������Cu2Sұ��ͭ����ȡCuSO4•5H2O������ͼ��
Cu2S�ǻ���ͭ����Ҫԭ��֮һ����������Cu2Sұ��ͭ����ȡCuSO4•5H2O������ͼ��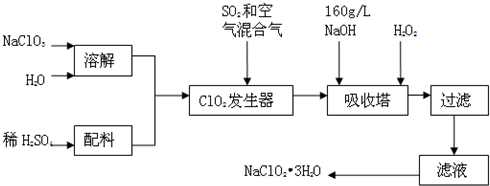
 ��˾ƥ�ֿ���ˮ�����������������Ƶã����Ʊ�ԭ�����£�
��˾ƥ�ֿ���ˮ�����������������Ƶã����Ʊ�ԭ�����£�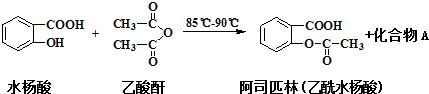

 ��
��