��Ŀ����
9����ͼ��ʵ�����Ʊ�����������һϵ�����ʵ���װ�ã��г��豸���ԣ���
��1���Ʊ�����ѡ�õ�ҩƷΪƯ�۾������Ũ���ᣬ��ط�Ӧ�Ļ�ѧ����ʽΪCa��ClO��2+4HCl��Ũ���TCaCl2+2Cl2��+2H2O��
��2��ʵ��װ����B�������dz������е��Ȼ��⣬��ȫƿ���ã�
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ������d�����ţ���
| ��� | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��5��װ��F�з�����Ӧ�����ӷ���ʽΪCl2+SO32-+H2O=SO42-+2H+�� ������������Һ�е�������������4%�������ʣ�������336mL������������Ҫ1mol•L-1�Ĵ�����������Һ156mL��
���� ��1��������ƾ���ǿ�������ԣ��ܹ�����Ũ���������������Ȼ��ơ�ˮ��
��2�������ӷ�����Ӧ��ȡ�������к����Ȼ��⣬װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ�����������������ʱB�еģ�ѹǿ����B�г���©����Һ���������γ�ˮ����
��3����֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�
��4����������װ��D��������Һ����װ��E�У����ɵ��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ���ܽ��ڱ��У��ֲ㣬�������ϲ㣻
��5���������ƾ���ǿ�Ļ�ԭ�ԣ��ܹ���������Ӧ������ԭ��Ӧ��������������������ӣ����ݷ���ʽ������Ҫ����������Һ�������
��� ��1��������ƾ���ǿ�������ԣ��ܹ�����Ũ���������������Ȼ��ơ�ˮ����ѧ����ʽ��Ca��ClO��2+4HCl��Ũ���TCaCl2+2Cl2��+2H2O��
�ʴ𰸣�Ca��ClO��2+4HCl��Ũ���TCaCl2+2Cl2��+2H2O��
��2�������ӷ�����Ӧ��ȡ�������к����Ȼ��⣬װ��B�б���ʳ��ˮ�������dz�ȥCl2�е�HCl��װ��B���ǰ�ȫƿ�����ʵ�����ʱC���Ƿ���������
�ʴ�Ϊ���������е��Ȼ��⣬��ȫƿ���ã�
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ���֤�����Ƿ����Ư���ԣ�Ҫ��֤����������Ư���ԣ�ʪ�����ɫ�����У�������ˮ��Ӧ���ɴ��������Ư���ԣ�ѡ����abc�Ģ��ж��Ǹ��������ͨ��ʪ�����ɫ����������֤������Ư���ԣ�����C��I��II��III���η���ʪ�����ɫ��������ˮ�Ȼ��ơ��������ɫ����������ѡd��
�ʴ�Ϊ��d��
��4����������װ��D�к��嵥�ʵ�������Һ�����ʵ⻯�غͱ���װ��E�У��嵥�ʺ͵⻯�ط�Ӧ���ɵⵥ�ʣ��ⵥ�����ڱ����Ϻ�ɫ�����۲쵽�������ǣ�E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ��
�ʴ�Ϊ��E����Һ��Ϊ���㣬�ϲ㣨���㣩Ϊ�Ϻ�ɫ��
��5���������ƾ���ǿ�Ļ�ԭ�ԣ��ܹ���������Ӧ������ԭ��Ӧ��������������������ӣ����ӷ���ʽ��Cl2+SO32-+H2O=SO42-+2H+��
336mL���������ʵ���Ϊ$\frac{0.336L}{22��4L/mol}$=0.015mol��
����Ҫ1mol•L-1�Ĵ�����������Һ���V�������ݷ���ʽ�ã�
Cl2+SO32-+H2O=SO42-+2H+��
1 1
0.015mol V��1mol•L-1����1-4%����
���V=0.0156L����156mL��
�ʴ�Ϊ��Cl2+SO32-+H2O=SO42-+2H+��156��
���� ���⿼��������ʵ������ȡ��������ѧ���ʡ�ʵ����ơ�ʵ��װ�õ��������ۡ���ѧ����ʽ�����ӷ���ʽ����д���йط���ʽ���㣬��Ŀ�Ѷ��еȣ�

| A�� | ������ɱ�����ˮ�����ռС�մ� | |
| B�� | ����ʣ����ᣬ���飬�ռʳ�� | |
| C�� | ͬ�������壺ʯī��C60��C80�����ʯ | |
| D�� | ����������������Ȫˮ�����������ᣬʯ��ʯ |
| A�� | ����������䣬����N2ʹ��ϵѹǿ���� | |
| B�� | �������������Сһ�� | |
| C�� | ��H2���� | |
| D�� | ѹǿ�������N2ʹ����������� |
| A�� | �����ơ�ƴ�����ĭ | |
| B�� | ��N2+3H2�T2NH3�ķ�Ӧ��ʹ������ý�ɼӿ�ϳɰ���Ӧ������ | |
| C�� | ��ҵ��ȡ������Na��l��+KCl��l���TNaCl��l��+K��g��ѡȡ���˵��¶ȣ�ʹK�������ӷ�Ӧ������з������ | |
| D�� | ��ˮ�д�������ƽ�⣺Cl2+H2O�THCl+HClO��������NaOH��Һ����ɫ��dz |
| A�� | ��ʯ���������� | B�� | �������ڵ���� | C�� | �������ڼ� | D�� | ���dz��������� |
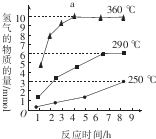 ����̼��COS������������������Ѭ������
����̼��COS������������������Ѭ������ ��
��