��Ŀ����
4��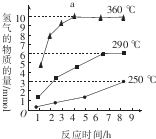 ����̼��COS������������������Ѭ������
����̼��COS������������������Ѭ��������1���������̼������ĸ�ԭ���У�ԭ�Ӱ뾶����Ԫ�������ڱ��е�λ���ǵ������ڵ�VA��
��2��������ʵ�����ڱȽ�C��P����Ԫ�طǽ��������ǿ������b������ĸ����
a����������ϼۣ�P��C
b��ͬ��ͬŨ�ȵ�����Һ�����ԣ�H3PO4��H2CO3
c���е㣺PH3��CH4
��3������̼ˮ�⼰����Ӧ���������£����ֲ�������ȥ����COS$��_{��}^{H_{2}O}$H2S$��_{��}^{NaOH��Һ}$Na2S$��_{��}^{��}$M��Һ+H2
����֪�������£���Ӧ����ÿ����1.7g H2S���壬��Ӧ�ų�����4.76kJ����÷�Ӧ���Ȼ�ѧ����ʽΪH2S��g��+2NaOH��aq��=Na2S��aq��+H2O��l����H=-95.2KJ/mol����֪M��Һ����Ԫ�ص���Ҫ������ʽΪS2O32-����Ӧ��������S2O32-�����ӷ���ʽΪ2S2-+5H2O$\frac{\underline{\;\;��\;\;}}{\;}$S2O32-+4H2��+2OH-��
����ͼ�Ƿ�Ӧ���У��ڲ�ͬ��Ӧ�¶��£���Ӧʱ����H2�����Ĺ�ϵ��Na2S��ʼ����Ϊ3mmol������ͼ�������֪��a��ʱM��Һ�г�S2O32-�⣬����SO42-������������ӷ��ţ���
���� ��1���������̼�������ԭ��ΪO��S��P��H�����жϵ��Ӳ��������Ӳ���Խ�࣬ԭ�Ӱ뾶Խ���Ӳ�����ͬ���ٸ���Ԫ�������ɣ�ͬ����Ԫ�ص�ԭ�Ӱ뾶����ԭ���������������С������Ԫ��������=���Ӳ�������������=������������
��2���Ƚ�����Ԫ�صķǽ�����ǿ�����ɸ��ݵ���֮����û���Ӧ����������Ӧ�����׳̶ȡ��⻯����ȶ����Լ���������������Ӧˮ��������ǿ����
��3���������Ȼ�ѧ����ʽ��д��������ע���ʵľۼ�״̬�Ͷ�Ӧ��Ӧ���ʱ�д���Ȼ�ѧ����ʽ��
�����������Ϣ�������Ӻ�ˮ��Ӧ���������������Ӻ����������ݵ���غ��ԭ���غ���д���ӷ�Ӧ����ʽ��
�۸���ͼʾ��Ӧʱ����H2�����Ĺ�ϵ��a��ʱM��Һ�г�S2O32-�⣬����SO42-��
��� �⣺��1���������̼�������ԭ��ΪO��S��P��H����Ԫ����1�����Ӳ㣬��Ԫ����2�����Ӳ㣬�ס�����3�����Ӳ㣬�����ס����ԭ�Ӱ뾶�����⡢����ԭ�Ӱ뾶���ס�����ͬ����Ԫ�أ����ԭ������������ԭ����������Ԫ��������֪����ԭ�Ӱ뾶�������ԭ�Ӱ뾶���������Ϊ5�����ӣ����ڵ������ڵ�VA�壬
�ʴ�Ϊ���������ڵ�VA�壻
��2���Ƚ�����Ԫ�صķǽ�����ǿ�����ɸ��ݵ���֮����û���Ӧ����������Ӧ�����׳̶ȡ��⻯����ȶ����Լ���������������Ӧˮ��������ǿ���ȽǶȣ�����������ϼۡ��⻯��ķе�ߵͲ������ڱȽ�Ԫ�صķǽ�����ǿ����
�ʴ�Ϊ��b��
��3���ٷ�Ӧ��Ϊ������������Ƶķ�ӦH2S+2NaOH=Na2S+H2O��1.7g H2S�����ʵ���Ϊn=$\frac{m}{M}$=$\frac{17g}{34g/mol}$=0.5mol����Ӧ�ų�����4.76kJ����1mol���ⷴӦ�ų�95.2KJ�����������Ը÷�Ӧ���Ȼ�ѧ����ʽΪH2S��g��+2NaOH��aq��=Na2S��aq��+H2O��l����H=-95.2KJ/mol��
�ʴ�Ϊ��H2S��g��+2NaOH��aq��=Na2S��aq��+H2O��l����H=-95.2KJ/mol��
�����������Ϣ�������Ӻ�ˮ��Ӧ���������������Ӻ�������������ԭ���غ㣬�������������ӣ�����ԭ���غ㣬���ﻹ�����������ӣ���ӦΪ��2S2-+5H2O$\frac{\underline{\;\;��\;\;}}{\;}$S2O32-+4H2��+2OH-��
�ʴ�Ϊ��2S2-+5H2O$\frac{\underline{\;\;��\;\;}}{\;}$S2O32-+4H2��+2OH-��
�۷�Ӧ���У�a��360��ʱ������ʱ�����ƣ������������䣬Na2S��ʼ����Ϊ3mmol����ֻ������2S2-+5H2O$\frac{\underline{\;\;��\;\;}}{\;}$S2O32-+4H2��+2OH-������������3mmol��$\frac{4}{2}$=6mmol��ͼ��Ϊ9mmol��˵��M��Һ�г�S2O32-�⣬����SO42-����ӦΪ��S2-+4H2O$\frac{\underline{\;\;��\;\;}}{\;}$SO42-+4H2����
�ʴ�Ϊ��SO42-��
���� ���⿼����ԭ�Ӱ뾶��С�ıȽϡ��ǽ�����ǿ���Ƚϡ��ȷ�Ӧ����ʽ����д�����ӷ�Ӧ����ʽ����д�ȣ�ע����Ӳ�ṹ��ͬ�����Ӱ뾶��С�ıȽϷ�����ע��Ԫ�طǽ����Ժͽ����ԵıȽϽǶȣ���Ŀ�Ѷ��еȣ�

 Сѧ������ҵϵ�д�
Сѧ������ҵϵ�д� ��ʿһ��ȫͨϵ�д�
��ʿһ��ȫͨϵ�д�| Ԫ�� | �����Ϣ |
| X | X�Ļ�̬ԭ�Ӻ���3���ܼ����е��ӣ���ÿ���ܼ��ϵĵ�������� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬���ڻ�ɽ�ڸ������� |
| Z | Z��Yͬ���ڣ�Z�ĵ縺�Դ���Y |
| W | W��һ�ֺ��ص�������Ϊ63��������Ϊ34 |
��2��XY2��һ�ֳ��õ��ܼ���XY2�ķ����д���2���Ҽ���X���ӻ����������sp��XY2�ķ��ӵĿռ乹��Ϊֱ���Σ���H-Y��H-Z���ֹ��ۼ��У������ϳ�����H-S��
��3��W�Ļ�̬ԭ�Ӻ�������Ų�ʽ��1s22s22p63s23p63d104s1��
��4��������XO��YO2�̵�����Ⱦ��һ�ַ������ǽ����ڴ���������ת��Ϊ����Y��
��֪��
XO��g��+0.5O2��g��=XO2��g����H=-283.0kJ/mol
Y��g��+O2��g��=YO2��g����H=-296.0kJ/mol
�˷�Ӧ���Ȼ�ѧ����ʽ��2CO��g��+SO2��g��=2CO2��g��+S��g����H=-270kJ/mol��
| A�� | ����Һ�м�KSCN����Һ�Ժ�ɫ��֤��ԭ��Һ����Fe3+����Fe2+ | |
| B�� | ����ͨ����ˮ����ͭ����ĩ������֤��ԭ�����к���ˮ���� | |
| C�� | ���հ�ɫ��ĩ������ɻ�ɫ��֤��ԭ��ĩ����Na+����K+ | |
| D�� | ������ͨ�����ʯ��ˮ����Һ����ǣ�֤��ԭ������CO2 |

��1���Ʊ�����ѡ�õ�ҩƷΪƯ�۾������Ũ���ᣬ��ط�Ӧ�Ļ�ѧ����ʽΪCa��ClO��2+4HCl��Ũ���TCaCl2+2Cl2��+2H2O��
��2��ʵ��װ����B�������dz������е��Ȼ��⣬��ȫƿ���ã�
��3��װ��C��ʵ��Ŀ������֤�����Ƿ����Ư���ԣ�Ϊ��C�Т����η������ʵ������d�����ţ���
| ��� | a | b | c | d |
| �� | �������ɫ���� | �������ɫ���� | ʪ�����ɫ���� | ʪ�����ɫ���� |
| �� | ��ʯ�� | �轺 | Ũ���� | ��ˮ�Ȼ��� |
| �� | ʪ�����ɫ���� | ʪ�����ɫ���� | �������ɫ���� | �������ɫ���� |
��5��װ��F�з�����Ӧ�����ӷ���ʽΪCl2+SO32-+H2O=SO42-+2H+�� ������������Һ�е�������������4%�������ʣ�������336mL������������Ҫ1mol•L-1�Ĵ�����������Һ156mL��

| A�� | N2��g��+3H2��g��?2NH3��g����H��0 | B�� | 2SO3��g��?2SO2��g��+O2��g����H��0 | ||
| C�� | 4NH3��g��+5O2��g��?4NO��g��+6H2O��g����H��0 | D�� | C��s��+H2O��g��?H2��g��+CO��g����H��0 |
| A�� | �ò�����պȡijδ֪��Һ������ɫ��Ӧ����������ɫΪ��ɫ��˵��һ������Ԫ�� | |
| B�� | ijδ֪��Һ��ϡ����������Һ��δ������ʹʪ���ɫʯ����ֽ���������壬��˵��ԭ��Һ����NH${\;}_{4}^{+}$ | |
| C�� | ij��Һ����CCl4��CCl4������ɫ��֤��ԭ��Һ�д���I- | |
| D�� | ij��Һ�������ᣬ�����������ټ�BaCl2��Һ��������ɫ��������˵����Һ��һ���� SO${\;}_{4}^{2-}$ |

�ش��������⣺
��1�����װ�������Եķ�����������D�ij��������ӵ��ܣ������ܵ�ĩ�˲���ˮ���е�ˮ�У��þƾ����������Բ����ƿ�������ܿ�������ð����ֹͣ���Ⱥ��ڵ������γ�һ��ˮ����˵��װ�����������ã�a�����������Ƿ�Һ©��
��2��д��NaNO2�ͣ�NH4��2SO4��Ӧ�Ʊ������Ļ�ѧ����ʽ2NaNO2+��NH4��2SO4$\frac{\underline{\;\;��\;\;}}{\;}$2N2��+Na2SO4+4H2O��
��3��װ��B�������dz�ȥ�����������������װ��C�������dz�ȥˮ������
��4����֪������Ļ�ѧ�����ȶ������������������������Һ����һ�ְ�ɫ�Ľ�״������һ���Σ���д���˻�ѧ��Ӧ����ʽ��Si3N4+4HF+9H2O=3H2SiO3��+4NH4F��
��5��ʵ������ijЩ�������ȡ���ռ���β������װ����ͼ2��ʾ��ʡ�Լгֺ;���װ�ã������ô�װ�úͱ����ṩ������������ʵ�飬�������ѡ����D
| ѡ�� | a���� | b���� | c�ռ������� | d�е����� |
| A | Ũ��ˮ | NaOH | NH3 | H2O |
| B | Ũ���� | Na2SO3 | SO2 | ����ʯ��ˮ |
| C | ϡ���� | Fe | NO2 | H2O |
| D | Ũ���� | KMnO4 | Cl2 | NaOH��Һ |
