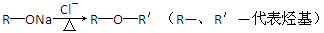��Ŀ����
CH4��H2��C�������ʵ���Դ���ʣ�����ȼ�յ��Ȼ�ѧ����ʽΪ��
��CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1��
��2H2(g)��O2(g)=2H2O(l)����H����571.6 kJ��mol��1��
��C(s)��O2(g)=CO2(g)����H����393.5 kJ��mol��1��
(1)����д���һ�ּ���ϸ������������øʹ������O2���ò���������������ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų�������________(����������������������)890.3 kJ��
(2)������CO2�����ںϳɺϳ���(��Ҫ�ɷ���һ����̼������)��CH4��CO2=2CO��2H2��1 g CH4��ȫ��Ӧ���ͷ�15.46 kJ����������
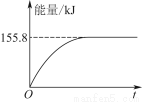
����ͼ�ܱ�ʾ�÷�Ӧ�����������仯����________(����ĸ)��
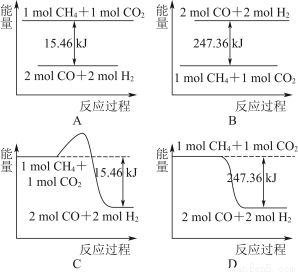
���������ʵ�����Ϊ1 mol��CH4��CO2����ij�����ܱ������У���ϵ�ų�����������ʱ��ı仯��ͼ��ʾ����CH4��ת����Ϊ________��
(3)C(s)��H2(g)����Ӧ������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����ֱ�Ӳ�������ͨ��������Ӧ�������C(s)��2H2(g)=CH4(g)�ķ�Ӧ����H��________��
(4)Ŀǰ���������������ʵ��о���ȼ���о����ص㣬���й��������������ʵ��о������п��е���________(����ĸ)��
A��Ѱ�����ʴ�����ʹCO2��H2O��Ӧ����CH4��O2�����ų�����
B��Ѱ�����ʴ������ڳ��³�ѹ��ʹCO2�ֽ�����̼��O2
C��Ѱ�����ʴ���������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2)
D������̬̼�ϳ�ΪC60����C60��Ϊȼ��
(1)����(2)��D����63%��(3)��74.8 kJ��mol��1��(4)C
��������(1)������Ӧ�ķ�Ӧ��ֻȡ���ڷ�Ӧ���������Ķ��ٺ�״̬�����м�����أ��ʼ���ϸ��ʹ1 mol��������CO2������Һ̬ˮ���ų��������Ե���890.3 kJ��
(2)��1 g CH4��ȫ��Ӧ�ͷ�15.46 kJ����������1 mol CH4��ȫ��Ӧ�ų�����Ϊ247.36 kJ����Dͼ�������⣻��CH4��ת���ʣ�155.8/247.36 ��100%��63%��
(3)��������������C(s)��2H2(g)=CH4(g)����H����74.8 kJ��mol��1��
(4)��֪CH4(g)��2O2(g)=CO2(g)��2H2O(l)����H����890.3 kJ��mol��1����CO2��H2O��Ӧ����CH4��O2�ķ�Ӧ���ȣ���A���ȷ��ʹCO2�ֽ�����̼��O2�ķ�ӦΪ���ȷ�Ӧ�������²��ܷ�������B���ȷ������̫����ʹ�����е�CO2�뺣���ɵ�CH4�ϳɺϳ���(CO��H2)�Ǻ����ģ�C����ȷ������̬̼�ϳ�ΪC60����C60��Ϊȼ�ϣ��������ú��㣬��D���ȷ

 ����ѧ��Ӧ�����ϵ�д�
����ѧ��Ӧ�����ϵ�д�̼���仯�����й㷺����;��
(1)��ˮ����ͨ�����ȵ�̼���ɲ���ˮú������ӦΪ
C(s)��H2O(g) CO(g)��H2(g)����H����131.3 kJ��mol��1��
CO(g)��H2(g)����H����131.3 kJ��mol��1��
���Ϸ�Ӧ�ﵽƽ������������������£����´�ʩ���������H2O��ƽ��ת���ʵ���________��(�����)
A�������¶� B������̼������ C��������� D����CO���ռ���ȥCO
(2)��֪��C(s)��CO2(g) 2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g)
2CO(g)����H����172.5 kJ��mol��1����CO(g)��H2O(g) CO2(g)��H2(g)���ʱ���H��________��
CO2(g)��H2(g)���ʱ���H��________��
(3)CO��H2��һ�������¿ɷ�Ӧ���ɼ״���CO(g)��2H2(g) CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ__________________________________��
CH3OH(g)���״���һ��ȼ�ϣ������ü״����һ��ȼ�ϵ�أ���ϡ�������������Һ�����ʯī���缫���õ�ظ�����ӦʽΪ__________________________________��
���øõ���ṩ�ĵ��ܵ��60 mL NaCl��Һ������0.01 mol CH3OH��ȫ�ŵ磬NaCl�������ҵ�������Cl2ȫ���ݳ������ǰ�������Һ����ı仯�����������������Һ��pH��________��
(4)��һ������CO(g)��H2O(g)�ֱ�ͨ�뵽���Ϊ2.0 L�ĺ����ܱ������У��������·�Ӧ��CO(g)��H2O(g) CO2(g)��H2(g)���õ��������ݣ�
CO2(g)��H2(g)���õ��������ݣ�
�¶�/�� | ��ʼ��/mol | ƽ����/mol | �ﵽƽ������ʱ��/min | ||
H2O | CO | H2 | CO |
| |
900 | 1.0 | 2.0 | 0.4 | 1.6 | 3.0 |
ͨ����������÷�Ӧ��ƽ�ⳣ��(���������λ��Ч����)________���ı䷴Ӧ��ijһ��������Ӧ���е�t minʱ����û��������CO2�����ʵ���Ϊ0.6 mol������200 mL 5 mol/L��NaOH��Һ������ȫ���գ���Ӧ�����ӷ���ʽΪ(��һ�����ӷ���ʽ��ʾ)__________________________
(5)��ҵ�����ǰ�ˮú���еĻ�����徭���������õĽϴ�H2���ںϳɰ����ϳɰ���Ӧԭ��ΪN2(g)��3H2(g) 2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
2NH3(g)����H����92.4 kJ��mol��1��ʵ����ģ�⻯���������ֱ��ڲ�ͬʵ�������·�Ӧ��N2Ũ����ʱ��仯��ͼ����ʾ��
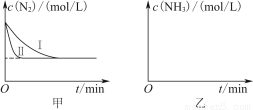
��ش��������⣺
����ʵ�����Ƚϣ�ʵ�����ı������Ϊ________________________________��
��ʵ������ʵ�������¶�Ҫ�ߣ�����������ͬ������ͼ���л���ʵ������ʵ������NH3Ũ����ʱ��仯��ʾ��ͼ��
��һ�ְ�ɫ��ĩ���������������Ӻ��������еļ��֡�
�����ӣ�S2����Cl����NO3����SO42����CO32����HCO3����MnO4����
�����ӣ�Na����Mg2����Al3����Ba2����Fe2����Fe3����Cu2����NH4+��
���ð�ɫ��ĩ��������ʵ�飬�۲쵽���������£�
ʵ����� | ���� |
a.ȡ������ĩ����ˮ���� | ȫ���ܽ⡢ |
��Һ��ɫ�� |
|
b.��������Һ�����������������Һ�������� | ���������� |
c.ȡ������ĩ�������� | ���������� |
d.ȡ������ĩ����ϡH2SO4��ϡHNO3�Ļ��Һ | �а�ɫ�������� |
����ʵ���ƶϣ�
(1)��aʵ���У����ƶϷ�ĩ�в�������______________(�����ӷ��ţ���ͬ)��
(2)��bʵ���У����ƶϷ�ĩ�в�������_____________________________________��
(3)��cʵ���У����ƶϷ�ĩ�в�������________________________________��
(4)��dʵ���У����ƶϷ�ĩ�в�������________��һ������________��
(5)���ϸ�ʵ������ȷ���Ƿ���ڵ�������____________��