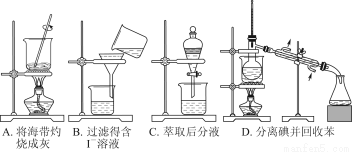
ЬтФПФкШн
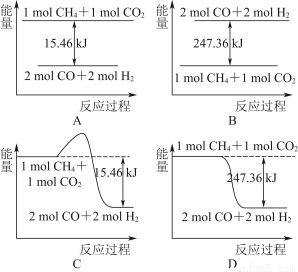
ФГПЮЭтЛюЖЏаЁзщЕФЭЌбЇЃЌбаОПгУЙ§СПЕФаПгыХЈСђЫсЗДгІжЦШЁЖўбѕЛЏСђЕФгаЙиЮЪЬтЃК
ЃЈ1ЃЉаПгыХЈСђЫсЗДгІжЦШЁЖўбѕЛЏСђЕФЛЏбЇЗНГЬЪНЮЊ______________________________ЁЃ
ЃЈ2ЃЉетбљжЦШЁЕФЖўбѕЛЏСђЦјЬхжаПЩФмКЌгаЕФдгжЪЪЧ__________________ЃЛдвђЪЧ________________________________________________________________________________________________________________________________________________ЁЃ
ЃЈ3ЃЉФГЭЌбЇгУЯТСазАжУСЌНгГЩвЛећЬзЪЕбщзАжУвдбщжЄЃЈ2ЃЉЕФХаЖЯЪЧЗёе§ШЗЃЌШєАДЦјЬхДгзѓЕНгвСїЯђЪБЃЌЦјЬхСїОЕФИїзАжУЕМЙмЕФБрКХвРДЮЪЧ________ЃЈгУaЁЂbЁЁЬюаДЃЉЁЃ
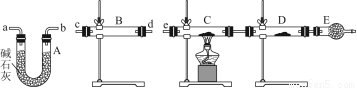
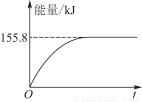
ЃЈ4ЃЉИљОнЃЈ3ЃЉШЗЖЈЕФЪЕбщЙ§ГЬЃЌЧыНЋЪЕбщЪБгаЙизАжУжаЫљЪЂвЉЦЗЁЂЪЕбщЯжЯѓЁЂНсТлЛђНтЪЭЬюШыЯТБэЃК
зАжУ | ЫљЪЂвЉЦЗ | ЪЕбщЯжЯѓ | НсТлЛђНтЪЭ |
B |
|
|
|
C | CuOЙЬЬх |
|
|
ЃЈ1ЃЉZnЃЋ2H2SO4ЃЈХЈЃЉ=ZnSO4ЃЋSO2ЁќЃЋ2H2O
ЃЈ2ЃЉЧтЦјКЭЫЎеєЦјЁЁЫцзХЗДгІЕФНјааХЈСђЫсБЛЯћКФЛсБфГЩЯЁСђЫсЃЌаПгыЯЁСђЫсЗДгІВњЩњЧтЦјЃЌЭЌЪБЛсгаЩйСПЫЎеєЦј
ЃЈ3ЃЉcdЃЈЛђdcЃЉЁЁabЃЈЛђbaЃЉЁЁe
ЃЈ4ЃЉ
зАжУ | ЫљЪЂвЉЦЗ | ЪЕбщЯжЯѓ | НсТлЛђНтЪЭ |
B | ЮоЫЎСђЫсЭ | ЙЬЬхгЩАзЩЋБфГЩРЖЩЋ | SO2жаКЌгаЫЎеєЦј |
C |
| ЙЬЬхгЩКкЩЋБфГЩКьЩЋ | SO2жаКЌгаЧтЦј |
ЁОНтЮіЁПЃЈ3ЃЉвЊМьбщSO2жаЕФЫЎеєЦјвдМАЧтЦјЃЌашЪзЯШгУЮоЫЎСђЫсЭМьбщЫЎеєЦјЃЌЧвгУМюЪЏЛвГ§ШЅЫЎеєЦјЃЌдйНЋЪЃгрЦјЬхвРДЮЭЈЙ§зЦШШЕФбѕЛЏЭКЭЮоЫЎСђЫсЭЃЌЭЈЙ§КкЩЋбѕЛЏЭБфКьКЭЮоЫЎСђЫсЭБфРЖЕФЯжЯѓжЄУїЦјЬхжаКЌгаЧтЦјЃЌзюКѓНЋЪЃгрЦјЬхгУЪЂгаМюЪЏЛвЕФИЩдяЙмЮќЪеЃЌЗРжЙЮлШОПеЦјЁЃ

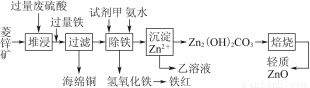
 ЧсЫЩЖсЙкШЋФмеЦПиОэЯЕСаД№АИ
ЧсЫЩЖсЙкШЋФмеЦПиОэЯЕСаД№АИСтаППѓЕФжївЊГЩЗжЪЧЬМЫсаПЃЌЛЙКЌгаЩйСПЕФFe2O3ЁЂFeOЁЂCuOЕШдгжЪЁЃШчЭМЪЧвдСтаППѓЮЊдСЯжЦШЁЧсжЪбѕЛЏаПЕФвЛжжЙЄвеСїГЬЃЌИУСїГЬЛЙПЩвдЕУЕНСНжжИБВњЦЗЁЊЁЊКЃУрЭКЭЬњКьЁЃ
ЧыНсКЯЯТБэЪ§ОнЃЌЛиД№ЮЪЬтЃК
Рызг | ПЊЪМГСЕэЪБЕФpH | ЭъШЋГСЕэЪБЕФpH |
Fe2ЃЋ | 6.3 | 9.7 |
Fe3ЃЋ | 1.5 | 3.2 |
Zn2ЃЋ | 6.2 | 8.0 |
ЃЈ1ЃЉЯТСаЪдМСжаЃЌ________ЃЈЬюДњКХЃЉПЩзїЮЊЪдМСМзЕФЪзбЁЁЃ
AЃЎKMnO4ЁЁ BЃЎCl2ЁЁ CЃЎH2O2ЁЁ DЃЎХЈЯѕЫс
ИљОнЫљбЁЪдМСаДГігыжЎЗДгІЕФРызгЗНГЬЪНЃК________________________________________________________________________________________________________________________________________________ЁЃ
ЃЈ2ЃЉЧтбѕЛЏЬњЙЬЬхЛсЮќИНвЛаЉSO42-ЃЌШчКЮЯДЕгFeЃЈOHЃЉ3ЙЬЬхвдМАШчКЮХаЖЯЪЧЗёЯДЕгИЩОЛЃП
ЯДЕгЗНЗЈЃК______________________________________________________________ЃЌХаЖЯЪЧЗёЯДЕгИЩОЛЕФЗНЗЈЪЧ_______________________________________ЁЃ
ЃЈ3ЃЉГ§ЬњВНжшжаМгШыАБЫЎЕФФПЕФЪЧЕїНкШмвКЕФpHЃЌЦфЪЪвЫЕФpHЗЖЮЇЪЧ______________ЃЛЕїНкШмвКpHЪБЃЌГ§СЫАБЫЎЭтЃЌЛЙПЩвдМгШыЯТСаЮяжЪжаЕФ________ЁЃ
aЃЎZnЁЁ bЃЎZnOЁЁ cЃЎZnЃЈOHЃЉ2ЁЁ dЃЎCuO
ЃЈ4ЃЉОВтЖЈввШмвКжаШдКЌгаЩйСПЕФFe3ЃЋКЭZn2ЃЋЁЃШєcЃЈFe3ЃЋЃЉЮЊ4.0ЁС10Ѓ17 molЁЄLЃ1ЃЌдђcЃЈZn2ЃЋЃЉЮЊ______________ molЁЄLЃ1ЁЃЃЈвбжЊKsp[FeЃЈOHЃЉ3]ЃН4.0ЁС10Ѓ38ЃЌKsp[ZnЃЈOHЃЉ2]ЃН1.2ЁС10Ѓ17ЃЉЁЃ
ЪвЮТЯТЃЌНЋвЛдЊЫсHAЕФШмвККЭKOHШмвКЕШЬхЛ§ЛьКЯ(КіТдЬхЛ§БфЛЏ)ЃЌЪЕбщЪ§ОнШчЯТБэЃК
ЪЕбщБрКХ | Ц№ЪМХЈЖШ/molЁЄLЃ1 | ЗДгІКѓШмвКЕФpH | |
c(HA) | c(KOH) | ||
Ђй | 0.1 | 0.1 | 9 |
Ђк | x | 0.2 | 7 |
ЯТСаХаЖЯВЛе§ШЗЕФЪЧ(ЁЁЁЁ)
AЃЎЪЕбщЂйЗДгІКѓЕФШмвКжаЃКc(KЃЋ)ЃОc(AЃ)ЃОc(OHЃ)ЃОc(HЃЋ)
BЃЎЪЕбщЂйЗДгІКѓЕФШмвКжаЃКc(OHЃ)ЃНc(KЃЋ)Ѓc(AЃ)ЃН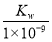 molЁЄLЃ1
molЁЄLЃ1
CЃЎЪЕбщЂкЗДгІКѓЕФШмвКжаЃКc(AЃ)ЃЋc(HA)ЃО0.1 molЁЄLЃ1
DЃЎЪЕбщЂкЗДгІКѓЕФШмвКжаЃКc(KЃЋ)ЃНc(AЃ)ЃОc(OHЃ)ЃНc(HЃЋ)