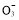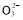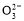��Ŀ����
�绯ѧԭ���ڹ�ҵ������������Ҫ�����ã���������ѧ֪ʶ�ش��й����⡣
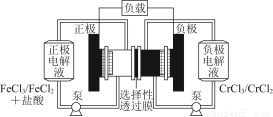
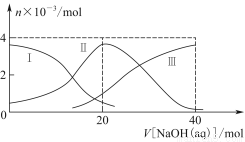
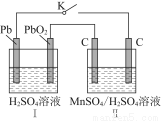
��1���õ��ķ�����������Һ����Ϊ��������о�������Ҫ��ʵ�����壬������ת��Ϊ�������ǵ�ⷨ�������������һ����Ҫ���ݡ���ͼ���ǵ������������ʵ��װ�ã�
����֪�����ķ�ӦΪ��x��1��S2��=Sx��S2����2xe�����������ĵ缫��Ӧʽ��________________________________________________________________________��
����Ӧת��x mol����ʱ���������������Ϊ____________����״���£���
����Na2S��9H2O����ˮ������������Һʱ��ͨ�����ڵ����������ܽ⡣��ԭ���ǣ������ӷ�Ӧ����ʽ��ʾ����___________________________________________________��
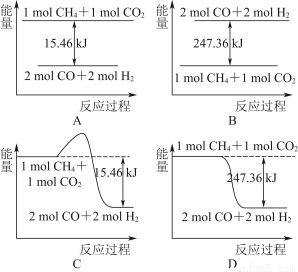
��2��MnO2��һ����Ҫ�������ܲ������Ʊ�MnO2�ķ���֮һ����ʯīΪ�缫������ữ��MnSO4��Һ�������ĵ缫��ӦʽΪ______________________������Ǧ����Ϊ��Դ����ữ��MnSO4��Һ����ͼ��ʾ��Ǧ���ص��ܷ�Ӧ����ʽΪ_______________________________________________������������4 mol H��������ʱ�����·��ͨ���ĵ��ӵ����ʵ���Ϊ________��MnO2�����۲���Ϊ________g��
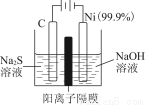
��3����ͼ���װ�ÿ��Ƶþ��о�ˮ���õ�FeO42-��ʵ������У������������������Y������Һ������FeO42-��
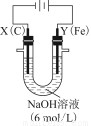
�ٵ������У�X������Һ��pH________����������������С����������������
���������У�Y�������ĵ缫��ӦΪFe��6e����8OH��=FeO42-��4H2O��________________________________________________________________________��
����X���ռ���672 mL���壬��Y���ռ���168 mL���壨��������Ϊ��״��ʱ�������������Y�缫�����缫����������________g��
��1����2H2O��2e��=2OH����H2������2H����2e��=H2������11.2x L
��2S2����O2��2H2O=2S����4OH��
��2��Mn2����2e����2H2O=MnO2��4H��
Pb��PbO2��2H2SO4=2PbSO4��2H2O
2 mol��87
��3������4OH����4e��=2H2O��O2����0.28
����������1�����ʱ��ˮ�����H�������������õ��ӻ�ԭ��Ӧ������H2�����ݵ����غ��֪��x mol����ת�ƣ�����H2 0.5x mol��S2�����н�ǿ��ԭ�ԣ��ױ������е�������������������Һʱ��Ҫ��������������
��2����������Mn2��ʧ��������MnO2�������е���Ԫ����Դ��ˮ������H�����ٽ��缫����ʽ��ƽ���ɡ�
��3��ͼ��X���ĵ缫��ӦΪ2H����2e��=H2������2H2O��2e��=H2����2OH����������X������pH��������������672 mL����֪�õ�����Ϊ0.06 mol��Y����������Ϊ168 mL��ʧ������0.03 mol���ɵ�ʧ�����غ��֪��ʧ������Ϊ0.03 mol���ɵ缫��Ӧ��֪���ܽ�Ϊ0.005 mol����0.28 g��

����ѪҺ��Ca2�����ӵ�Ũ��һ�����mg/mL����ʾ���������IJ����[(NH4)2C2O4]��Һ�������������(CaC2O4)���������˲���Ƴ���ϴ�Ӻ�����ǿ��ɵò���(H2C2O4)������KMnO4��Һ�ζ���ʹ����ת����CO2�ݳ���
�Իش�
(1)����Ҫ80 mL 0.02 mol��L��1��KMnO4��Һ�����еζ�����������Һʱ��Ҫ�IJ����������ձ�����������______________________������ʱ�� KMnO4��ҺӦ��ǿ���ữ����ʵ��ѡ��________���ữ������ѡ��HNO3�ữ����������________(����ƫ������ƫС������������)��
(2)������KMnO4��Ӧ�����ӷ���ʽΪ______________________________________
(3)�ζ�ʱ����������___________________________________
����ȷ����Ӧ�ﵽ�յ㡣
(4)�ζ���ʵ������������ʾ��
ʵ���� | ����ѪҺ�����/mL | ����KMnO4��Һ�����/mL |
1 | 20.00 | 11.95 |
2 | 20.00 | 13.00 |
3 | 20.00 | 12.05 |
�������㣬ѪҺ��Ʒ��Ca2�����ӵ�Ũ��Ϊ________mg/mL��
(5)�ζ��ķ���������к͵ζ��������ζ�����ϵζ��ȡ������ζ����õ�ָʾ����������һ�ֳ���������AgNO3����Һ�ⶨCl��Ϊ����
�յ�ǰ��Ag����Cl��=AgCl (��ɫ)
�յ�ʱ��2Ag����CrO42��=Ag2CrO4(ש��ɫ)
������ΪAgCl�ܽ�ȱ�Ag2CrO4��________��Ե�ʡ�