��Ŀ����
298K�¶��£�CuSO4?5H2O��ˮ��ƽ��ѹ��Ϊ��
ƽ���ϵ��������p��H2O����1.04kPa��CuSO4?5H2O���ֽ⣻p��H2O��=1.04kPa����CuSO4?5H2O��CuSO4?3H2O����ƽ�⣮��������
| ��ѧ��Ӧ | p��H2O��/kPa |
| ��1��CuSO4?5H2O��s��?CuSO4?3H2O��s��+2H2O��g�� | 1.04 |
| ��2��CuSO4?3H2O��s��?CuSO4?H2O��s��+2H2O��g�� | 0.75 |
| ��3��CuSO4?H2O��s��?CuSO4��s��+H2O��g�� | 0.11 |
| A��p��H2O��=0.75 kPaʱ��CuSO4?3H2O���ֽ� |
| B��p��H2O����0.11 kPaʱ��CuSO4?H2O��CuSO4����ƽ�� |
| C��0.75kPa��p��H2O����0.11kPaʱ����CuSO4?H2O���ȶ��� |
| D��1.04kPa��p��H2O����0.75kPaʱ����ϵ�в�����CuSO4?5H2O��CuSO4?H2O��CuSO4 |
���㣺��ѧƽ���Ӱ������
ר�⣺��ѧƽ��ר��
������ƽ���ϵ������298Kʱ������p��H2O����1.04kPa��CuSO4?5H2O���ֽ⣻p��H2O��=1.04kPa����CuSO4?5H2O��CuSO4?3H2O����ƽ�⣻
1.04kPa��p��H2O����0.75kPa����CuSO4?3H2O�ȶ�������û��CuSO4?5H2O��CuSO4?H2O��CuSO4��
p��H2O��=0.75 kPa��CuSO4?3H2O��CuSO4?H2O����ƽ�⣮
1.04kPa��p��H2O����0.75kPa����CuSO4?3H2O�ȶ�������û��CuSO4?5H2O��CuSO4?H2O��CuSO4��
p��H2O��=0.75 kPa��CuSO4?3H2O��CuSO4?H2O����ƽ�⣮
���
�⣺A��p��H2O��=0.75 kPaʱ��CuSO4?3H2O��s��?CuSO4?H2O��s��+2H2O��g����CuSO4?3H2O�ֽ⣬��A����
B��p��H2O��=0.11 kPaʱ��CuSO4?H2O��CuSO4����ƽ�⣬��B����
C������ͼ�����ݷ�����֪��0.75kPa��p��H2O����0.11kPaʱ����CuSO4?H2O���ȶ�������C��ȷ��
D��1.04kPa��p��H2O����0.75kPa����CuSO4?3H2O�ȶ�������û��CuSO4?5H2O��CuSO4?H2O��CuSO4����D��ȷ��
��ѡCD��
B��p��H2O��=0.11 kPaʱ��CuSO4?H2O��CuSO4����ƽ�⣬��B����
C������ͼ�����ݷ�����֪��0.75kPa��p��H2O����0.11kPaʱ����CuSO4?H2O���ȶ�������C��ȷ��
D��1.04kPa��p��H2O����0.75kPa����CuSO4?3H2O�ȶ�������û��CuSO4?5H2O��CuSO4?H2O��CuSO4����D��ȷ��
��ѡCD��
���������⿼������Ϣ�����ж��������������ʵ�����Ӧ�ã����ջ����ǹؼ�����Ŀ�ѶȽϴ�

��ϰ��ϵ�д�
 ��ս100��Ԫ����Ծ�ϵ�д�
��ս100��Ԫ����Ծ�ϵ�д�
�����Ŀ
 ������ͼ��ʾ���ܽ�ȱ���Ҫ�ӻ���Na2SO4��Na2Cr2O7�����еõ�Na2Cr2O7����Ҫ������ز�������Щ������������������
������ͼ��ʾ���ܽ�ȱ���Ҫ�ӻ���Na2SO4��Na2Cr2O7�����еõ�Na2Cr2O7����Ҫ������ز�������Щ������������������| A�����½ᾧ | B�����ȹ��� |
| C�����½ᾧ | D�������ᾧ |
�����й��л���ͬ���칹��˵���в���ȷ���ǣ�������
| A������ʽΪC4H8��ͬ���칹�干�У������������칹��5�� |
| B�������飨C8H8��������ȡ������3�� |
| C������ʽΪC5H12O��ͬ���칹�������ڴ������7�� |
D�� ��һ��ȡ������4�� ��һ��ȡ������4�� |
�����йػ�ѧ��Ӧ���ʵ�˵���У���ȷ���ǣ�������
| A��100mL 2 mol/L��������п��Ӧʱ�������������Ȼ�����Һ���������������ʲ��� |
| B������Ƭ��ϡ���ᷴӦ��ȡ����ʱ��������Ƭ��Ũ������Լӿ�������������� |
| C����������Ĵ�������һ�����ȷ�Ӧ�����������¶ȣ���Ӧ���ʼ��� |
| D������β���е�CO��NO���Ի�����Ӧ����N2��CO2����Сѹǿ����Ӧ���ʼ��� |
��֪��2KMnO4+16HCl=2KCl+2MnCl2+5Cl2+8H2O��2FeCl2+Cl2=2FeCl3���������ʵ���������ǿ������˳���ǣ�������
| A��KMnO4��Cl2��FeCl3 |
| B��Cl2��KMnO4��FeCl3 |
| C��FeCl3��Cl2��KMnO4 |
| D��FeCl3��KMnO4��Cl2 |
�й�Na2CO3��NaHCO3�����ʣ���������������ǣ�������
| A����������Na2CO3��NaHCO3�����������ᷴӦ������ͬ������NaHCO3������CO2��������� |
| B��ͬһ�¶��£����Ũ�ȵ����ᷴӦʱ��NaHCO3��Na2CO3���� |
| C����ͬ���ᷴӦ���ɵ�����CO2ʱ�������ĵ��������� |
| D����ʯ��ˮ���뵽NaHCO3��Һ�н���������� |
����װ�û�������ܴﵽʵ��Ŀ���ǣ�������
A��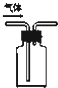 �����ſ������ռ�CO2 �����ſ������ռ�CO2 |
B��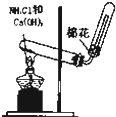 ʵ������ȡNH3 ʵ������ȡNH3 |
C��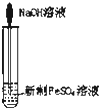 ʵ������ȡFe��OH��2 ʵ������ȡFe��OH��2 |
D��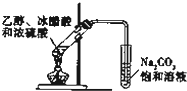 ʵ�������������� ʵ�������������� |