��Ŀ����
����Ŀ��ij��ѧѧϰС�����ʵ����ȡ������̽���������й����ʣ�
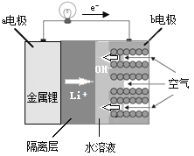
(1)ʵ�����Ʊ����������з���������ѡ�õ��� ___________(����)
A . �Ȼ����Һ������������Һ����
B. �������������еμ�Ũ��ˮ
C. ��̬�Ȼ�識��ȷֽ�
D. ��̬����識��ȷֽ�
E. ��̬�Ȼ������ʯ�һ�ϼ���
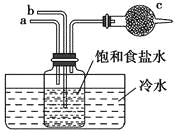
(2)��С���Ա���ʵ���Ʊ�������̽�������Ļ�ԭ�Լ��������ṩʵ��װ����ͼ��
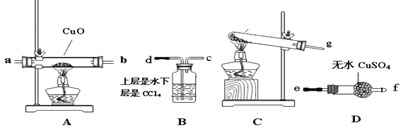
��װ��C�з�����Ӧ�Ļ�ѧ����ʽΪ______________
�ڸ��������ṩ��װ����ȷ������˳��Ϊ____________(�ø��ӿ���ű�ʾ)
�۸�װ�����������һ����ȱ�ݣ���Ը�װ�õĸĽ���ʩ��___________��
(3)���øĽ����װ�ý���ʵ�飬�۲쵽CuOȫ����Ϊ��ɫ���ʣ���ˮCuSO4������ͬʱ����һ������Ⱦ�����塣��д��NH3��CuO��Ӧ�Ļ�ѧ����ʽ________
(4)��ͬѧ��Ϊ��NH3��CuO��Ӧ���ɵĺ�ɫ�����п��ܺ���Cu2O���������һ����ʵ�����ú�ɫ�������Ƿ���Cu2O__________����֪��Cu2O+2H+=Cu+Cu2+ +H2O
���𰸡�BE 2NH4Cl+Ca(OH)2![]() CaCl2+2NH3��+2H2O g��ab��ef��d ��װ��C��A֮������һ��ʢ�м�ʯ�ҵĸ���� 3CuO+2NH3=3Cu+N2+3H2O ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵����ɫ�����к���Cu2O����֮����û��
CaCl2+2NH3��+2H2O g��ab��ef��d ��װ��C��A֮������һ��ʢ�м�ʯ�ҵĸ���� 3CuO+2NH3=3Cu+N2+3H2O ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵����ɫ�����к���Cu2O����֮����û��
��������
(1)ʵ�����г����Ȼ�粒�����������ƹ��干�ȣ��Լ�Ũ��ˮ���������ƹ������ʯ�ҹ��ȣ���ȡ������
(2) ����ʵ��װ�ð��ա�����ȡ����������ԭ����֤����������β����������������õĹ�������������ȡ��������������Ȼ�狀��������ơ�
(1)A���Ȼ����Һ������������Һ���ȣ����ɰ�����������������ˮ���÷������ʺ��ư�����A����
B���������������еμ�Ũ��ˮ������������ˮ���ų������ȣ��ٽ�NH3��H2O�ķֽ⼰NH3�Ļӷ��ݳ����ʺ��ư�����B��ȷ��
C����̬�Ȼ�識��ȷֽ����ɰ������Ȼ��⣬���������Ȼ���һ����ȴ�����ֿ��Ի��ϳ��Ȼ�泥����ܷ���װ�ö������÷��������ư�����C����
D����̬����識��ȷֽ⣬������ը���÷�����ʺ��ư�����D����
E����̬�Ȼ������ʯ�һ�ϼ��ȿ�����ȡ������ԭ��Ϊ��2NH4Cl+Ca(OH)2 ![]() CaCl2+2NH3��+2H2O��E��ȷ��
CaCl2+2NH3��+2H2O��E��ȷ��
��ΪBE��
(2)��װ��C��ʵ�����ù�����ʯ�Һ��Ȼ�淋Ļ������ȡ������װ�ã���Ӧ�Ļ�ѧ����ʽΪ2NH4Cl+Ca(OH)2 ![]() CaCl2+2NH3��+2H2O��
CaCl2+2NH3��+2H2O��
��װ��d����˳��Ϊ����ȡ����������ԭ����֤����������β������������װ����ȷ�Ľӿ�����˳��Ϊg��ab��ef��d��
�۰�����ԭCuO����ˮ��Ϊ��ֹ�����л���ˮ�ĸ��ţ���ȡ�İ�������������ѡ�����Ը��������ˮ�Ȼ��ƣ���ѡ�ü�ʯ�һ���ʯ�Ҹ������װ��C��A֮������һ��ʢ�м�ʯ�ҵĸ���ܣ�
(3)������ԭCuO������Ϊ����ɫ����ȫ����Ϊ��ɫ���ʣ���ˮCuSO4������ͬʱ����һ������Ⱦ�����壬��������ԭCuO����H2O��Cu��N2����ѧ����ʽΪ3CuO+2NH3 ![]() 3Cu+N2+3H2O��
3Cu+N2+3H2O��
(4)������Ϣ��Cu2O+2H���TCu+Cu2��+H2O��֪��Cu2O����ϡ���ᡢHCl�ȷ��������ᷴӦ��������ɫ��Һ��������Ƶ�ʵ�鷽��Ϊ��ȡ������Ʒ������ϡH2SO4������Һ������ɫ��˵����ɫ�����к���Cu2O��

����Ŀ������ʵ���������������۶�Ӧ��ϵ��ȷ����
ѡ�� | ʵ����� | ʵ������ | ���� |
A | ��ͬ�¶��£�ͬʱ�� ��4 mL 0.1 molL-1 KMnO4������Һ����4 mL 0.2 molL-1 KMnO4������Һ�У��ֱ����4 mL 1 molL-1 H2C2O4��Һ | ������Һ����ɫ | ��ʵ�������£�KMnO4Ũ��ԽС����Ӧ����Խ�� |
B | ��ú¯�����ȵ�ú̿��������ˮ | ��������ɫ���棬ú̿ȼ�ո��� | ������ˮ��ʹú̿ȼ�շų���������� |
C | ����2NO2(g) | ��ɫ���� | ֤������Ӧ�Ƿ��ȷ�Ӧ |
D | �ֱ�ⶨ�����µ����ʵ���Ũ�ȵ�Na2SO3��Na2CO3��Һ��pH | ���߽ϴ� | ֤���ǽ����� S��C |
A.AB.BC.CD.D