题目内容
【题目】工业上用含三价钒(V2O3)为主的某石煤为原料(含有Al2O3、CaO等杂质),钙化法焙烧制备V2O5,其流程如下:
![]()
资料:+5价钒在溶液中的主要存在形式与溶液pH的关系:
pH | 4~6 | 6~8 | 8~10 | 10~12 |
主要离子 | VO2+ | VO3— | V2O74— | VO43— |
(1)焙烧:向石煤中加生石灰焙烧,将V2O3转化为Ca(VO3)2的化学方程式是_________。
(2)酸浸:①Ca(VO3)2难溶于水,可溶于盐酸。若焙砂酸浸时溶液的pH=4,Ca(VO3)2溶于盐酸的离子方程式是________。
②酸度对钒和铝的溶解量的影响如图所示:酸浸时溶液的酸度控制在大约3.2%,根据下图推测,酸浸时不选择更高酸度的原因是________。

(3)转沉:将浸出液中的钒转化为NH4VO3固体,其流程如下:
![]()
①浸出液中加入石灰乳的作用是_____________。
②已知常温下CaCO3的溶度积常数为Ksp1,Ca3(VO4)2溶度积常数为Ksp2。过滤后的(NH4)3VO4溶液中VO43—的浓度为cmol/L,该溶液中CO32—的浓度为_______mol/L
③向(NH4)3VO4溶液中加入NH4Cl溶液,控制溶液的pH=7.5。当pH>8时,NH4VO3的产量明显降低,原因是_______________。
(4)测定产品中V2O5的纯度:称取ag产品,先用硫酸溶解,得到(VO2)2SO4溶液。再加入b1mLc1mol/L(NH4)2Fe(SO4)2溶液(VO2++2H++Fe2+=VO2++Fe3++H2O)最后用c2mol/LKMnO4溶液滴定过量的(NH4)2Fe(SO4)2至终点,消耗KMnO4溶液的体积为b2mL。
已知MnO4-被还原为Mn2+,假设杂质不参与反应。则产品中V2O5的质量分数是_____。(V2O5的摩尔质量:182g/mol)
【答案】CaO+O2+V2O3![]() Ca(VO3)2 Ca(VO3)2+4H+=2
Ca(VO3)2 Ca(VO3)2+4H+=2![]() +Ca2++2H2O 酸度大于3.2%时,钒的溶解量增大不明显,而铝的溶解量增大程度更大 调节溶液的pH,并提供Ca2+,形成Ca3(VO4)2沉淀
+Ca2++2H2O 酸度大于3.2%时,钒的溶解量增大不明显,而铝的溶解量增大程度更大 调节溶液的pH,并提供Ca2+,形成Ca3(VO4)2沉淀 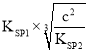 当pH>8时,钒的主要存在形式不是
当pH>8时,钒的主要存在形式不是![]()
![]()
【解析】
焙烧过程将V2O3转化为Ca(VO3)2,用盐酸酸浸,浸出液中含有VO2+、Al3+、Ca2+、Cl-、H+,加入石灰乳调节pH得到Ca3(VO4)2沉淀,由表中数据,可知应控制pH范围为10~12,由Ca(VO3)2![]() Ca3(VO4)2,可知氢氧化钙还提供Ca2+。过滤分离,Ca3(VO4)2与碳酸铵反应转化为更难溶的CaCO3沉淀,c(Ca2+)降低,使钒从沉淀中转移到溶液中形成(NH4)3VO4溶液,溶液中加入NH4Cl,调节溶液pH,同时溶液中NH4+增大,有利于析出(NH4)3VO3;据此分析解答。
Ca3(VO4)2,可知氢氧化钙还提供Ca2+。过滤分离,Ca3(VO4)2与碳酸铵反应转化为更难溶的CaCO3沉淀,c(Ca2+)降低,使钒从沉淀中转移到溶液中形成(NH4)3VO4溶液,溶液中加入NH4Cl,调节溶液pH,同时溶液中NH4+增大,有利于析出(NH4)3VO3;据此分析解答。
(1)焙烧过程将V2O3转化为Ca(VO3)2,CaO参与反应,V元素化合价升高,需要氧气参加反应,反应方程式为:CaO+O2+V2O3![]() Ca(VO3)2,故答案为:CaO+O2+V2O3
Ca(VO3)2,故答案为:CaO+O2+V2O3![]() Ca(VO3)2;
Ca(VO3)2;
(2)①Ca(VO3)2难溶于水,可溶于盐酸,若焙砂酸浸时溶液的pH=4,由表中数据可知,Ca(VO3)2溶于盐酸转化为VO2+,反应离子方程式为:Ca(VO3)2+4H+═2VO2++Ca2++2H2O,故答案为:Ca(VO3)2+4H+=2![]() +Ca2++2H2O;
+Ca2++2H2O;
②根据如图推测,酸浸时不选择更高酸度的原因是:酸度大于3.2%时,钒的溶解量增大不明显,而铝的溶解量增大程度更大;故答案为:酸度大于3.2%时,钒的溶解量增大不明显,而铝的溶解量增大程度更大;
(3)焙烧过程将V2O3转化为Ca(VO3)2,用盐酸酸浸,浸出液中含有VO2+、Al3+、Ca2+、Cl-、H+,加入石灰乳调节pH得到Ca3(VO4)2沉淀,由表中数据,可知应控制pH范围为10~12,由Ca(VO3)2![]() Ca3(VO4)2,可知氢氧化钙还提供Ca2+。过滤分离,Ca3(VO4)2与碳酸铵反应转化为更难溶的CaO3沉淀,c(Ca2+)降低,使钒从沉淀中转移到溶液中形成(NH4)3VO4溶液,溶液中加入NH4Cl,调节溶液pH,同时溶液中NH4+增大,有利于析出(NH4)3VO3;
Ca3(VO4)2,可知氢氧化钙还提供Ca2+。过滤分离,Ca3(VO4)2与碳酸铵反应转化为更难溶的CaO3沉淀,c(Ca2+)降低,使钒从沉淀中转移到溶液中形成(NH4)3VO4溶液,溶液中加入NH4Cl,调节溶液pH,同时溶液中NH4+增大,有利于析出(NH4)3VO3;
①浸出液中加入石灰乳的作用是:调节溶液的pH,并提供Ca2+,形成Ca3(VO4)2沉淀;故答案为:调节溶液的pH,并提供Ca2+,形成Ca3(VO4)2沉淀;
②根据Ksp的表达式计算得: ,故答案为:
,故答案为: ;
;
③当pH>8时,钒的主要存在形式不是VO3-,NH4VO3的产量明显降低,故答案为:当pH>8时,钒的主要存在形式不是![]() ;
;
(4)加入高锰酸钾发生反应:5Fe2++MnO4-+8H+=Mn2++5Fe3++4H2O,反应消耗KMnO4为:b2×10-3L×c2molL-1=b2c2×10-3mol,剩余的Fe2+物质的量为:5b2c2×10-3mol,与VO2+反应Fe2+物质的量为:b1×10-3L×c1molL-1-5 b2c2×10-3mol=(b1c1-5b2c2)×10-3mol,由关系式:V2O5~2NH4VO3~2VO2+~2Fe2+,可知n(V2O5)=![]() (b1c1-5b2c2)×10-3mol,故产品中V2O5的质量分数为:
(b1c1-5b2c2)×10-3mol,故产品中V2O5的质量分数为: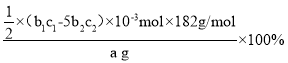 =
=![]() ,故答案为:
,故答案为:![]() 。
。

【题目】钼及其合金在冶金、电器等方面有广泛应用。在1L恒容密闭容器中充入足量的Na2CO3、MoS2和H2,发生反应:MoS2(s)+2Na2CO3(s)+4H2(g)Mo(s)+2CO(g)+4H2O(g)+2Na2S(s) △H,测得在不同温度下H2的物质的量与时间关系数据如表所示:下列说法错误的是( )
min mol K | 0 | 10 | 20 | 30 | 40 |
T1 | 2 | 1.6 | 1.3 | 1.0 | 1.0 |
T2 | 2 | 1.2 | 1.0 | n | 0.6 |
A.T2>T1,△H<0
B.表格中n=0.6
C.T2K下20min时,v正>v逆
D.T1K下平衡常数K=0.25(molL-1)2