��Ŀ����
15�� �о���ѧϰ������ѧ������˼ά�����÷�����ij�о���ѧϰС�齫����װ����ͼ���ӣ�C��D��E��F���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ��
�о���ѧϰ������ѧ������˼ά�����÷�����ij�о���ѧϰС�齫����װ����ͼ���ӣ�C��D��E��F���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ���ش����⣺
��1����ԴA����������������
��2����װ���е�ⷴӦ���ܻ�ѧ����ʽ��2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2SO4��
��3������ռ���װ���в��������壬����������������1��1��
��4�����ñ�װ�ø�ͭ������GӦ����Ag���ͭ���������������Һ����Ҫ�ɷ���AgNO3���ѧʽ����
��5��װ������F���ĵ缫��Ӧ��2H2O+2e-=H2��+2OH-��
���� ��װ���ǵ��أ�C��D��E��F���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ��˵��F�缫������Һ�ʼ��ԣ���NaOH���ɣ���F��������E������������C��E��G��������D��F��H��������
��1�����������ĵ缫�����������������ĵ缫�Ǹ�����
��2���ö��Ե缫�������ͭ��Һʱ��������ˮʧ�������������������ӣ�������ͭ���ӷŵ�����Cu��
��3����ⱥ��ʳ��ˮʱ��������������Ӧʽ�ֱ�Ϊ2Cl--2e-=Cl2����2H2O+2e-=H2��+2OH-��������·��ת�Ƶ�����ȣ�
��4�����ʱ���Ʋ����������Ƽ����������������Һ�н��������Ӻ���������Ϊͬһ����Ԫ�أ�
��5��F�缫��ʧ�����������������������ӣ�
��� �⣺��װ���ǵ��أ�C��D��E��F���Ƕ��Ե缫������Դ��ͨ�������е����̪��Һ����F�������Ժ�ɫ��˵��F�缫������Һ�ʼ��ԣ���NaOH���ɣ���F��������E������������C��E��G��������D��F��H��������
��1�����������ĵ缫�����������������ĵ缫�Ǹ���������A��������B�Ǹ������ʴ�Ϊ��������
��2���ö��Ե缫�������ͭ��Һʱ��������ˮʧ�������������������ӣ�������ͭ���ӷŵ�����Cu����ط�ӦʽΪ2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2SO4��
�ʴ�Ϊ��2CuSO4+2H2O$\frac{\underline{\;���\;}}{\;}$2Cu��+O2��+2H2SO4��
��3����ⱥ��ʳ��ˮʱ��������������Ӧʽ�ֱ�Ϊ2Cl--2e-=Cl2����2H2O+2e-=H2��+2OH-��������·��ת�Ƶ�����ȣ����ݵ缫��Ӧʽ֪�������缫������������ʵ�����ȣ���ͬ�������������֮��Ϊ1��1���ʴ�Ϊ��1��1��
��4�����ʱ���Ʋ����������Ƽ����������������Һ�н��������Ӻ���������Ϊͬһ����Ԫ�أ����ñ�װ�ø�ͭ��������GΪAg��HΪCu���������ҺΪAgNO3��Һ���ʴ�Ϊ��Ag��AgNO3��
��5��F�缫��ʧ�����������������������ӣ��缫��ӦʽΪ2H2O+2e-=H2��+2OH-���ʴ�Ϊ��2H2O+2e-=H2��+2OH-��
���� ���⿼����ԭ�����漰���͵�ƣ���ȷ�ж����������������ǽⱾ��ؼ���֪�������缫�Ϸ����ķ�Ӧ������ȷ��д�缫��Ӧʽ����Ŀ�ѶȲ���

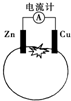
| A�� | һ��ʱ���пƬ��������� | B�� | ͭ�缫�����������ɫ | ||
| C�� | ������ͭͨ����������п | D�� | п�缫�Ǹõ�صĸ��� |
| A�� | ��X��Y����ͬһ���ڣ�����M�����������������ͷ��� | |
| B�� | ��X�ĵ��ʳ����������壬��Y�ĵ��ʳ�����Ҳ������ | |
| C�� | ��X��Y��Ԫ����������1�����������ӻ������Mֻ������ | |
| D�� | ��M�������ӻ���������п��ܺ��зǼ��Լ� |
| A�� | ��������������ϡ���Fe3O4+8H+=Fe2++2Fe3++4H2O | |
| B�� | Ca��HCO3��2�����Ca��OH��2��Һ��Ӧ��Ca2++HCO3-+OH-=CaCO3��+H2O | |
| C�� | ������ͭ�����ϡ���2H++2Cl-$\frac{\underline{\;���\;}}{\;}$H2��+Cl2�� | |
| D�� | Na2S2O3��Һ�м���ϡ���2S2O+4H+�TSO42-+3S��+2H2O |
| A�� | �� CH3��2CHCH2CH2CH3 | B�� | ��CH3CH2��2CHCH3 | C�� | ��CH3��2CHCH��CH3��2 | D�� | ��CH3��3CCH3 |
| Ԫ�� | A | B | C | D | E | F | G | H |
| ԭ�Ӱ뾶��nm�� | 0.160 | 0.143 | 0.089 | 0.102 | 0.074 | 0.152 | 0.037 | 0.099 |
| ��Ҫ���ϼ� | +2 | +3 | +2 | +6��-2 | -2 | +1 | +1��-1 | +7��-1 |
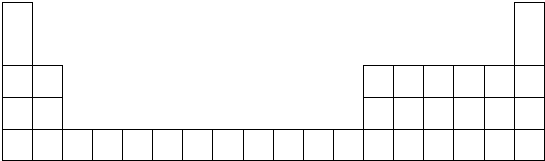
��2��C��H��Ԫ�ص��������������Ӧ��ˮ�������Ӧ�����ӷ���ʽ��Be��OH��2+2H+�TBe2++2H2O��
��3��F��E��ȼ�յĻ�ѧ��Ӧ����ʽ��4Li+O2$\frac{\underline{\;��ȼ\;}}{\;}$2Li2O
��4����Ƽ��Թ�ʵ��֤��DԪ����HԪ�صķǽ�����ǿ����ϵ������ˮ����������Һ�У����е���ɫ�������ɿ�֤�����������Դ���S���Ӷ�֤���ǽ�����Cl��S��
 ��̼Ԫ�ص��⻯���CH4�⣬����C2H6����Ԫ�ص��⻯���NH3�⣬���к�2����ԭ�ӵķ��ӵĻ�ѧʽΪN2H4����е�Ȱ����ߣ���ߡ��͡��������⻯�����������ᷴӦ�Ļ�ѧ��Ӧ����ʽΪN2H4+2HCl=N2H6Cl2��
��̼Ԫ�ص��⻯���CH4�⣬����C2H6����Ԫ�ص��⻯���NH3�⣬���к�2����ԭ�ӵķ��ӵĻ�ѧʽΪN2H4����е�Ȱ����ߣ���ߡ��͡��������⻯�����������ᷴӦ�Ļ�ѧ��Ӧ����ʽΪN2H4+2HCl=N2H6Cl2��
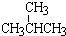 ��
�� �� ��
�� ��
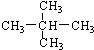 ��
��