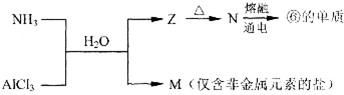
��Ŀ����
8��I���£�N2H4���ֳ��������ڳ�������һ�ֿ�ȼ�Ե�Һ�壬���������ȼ�ϣ���֪��25�桢l0lkPaʱ��16gN2H4����������ȫȼ�����ɵ�����H2O���ų�����312kJ����1���������£�N2H4��ȫȼ�յ��Ȼ�ѧ����ʽΪN2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��
��2����ͳ�Ʊ��µķ�������NaClO����NH3���Ƶ��µ�ϡ��Һ���÷�Ӧ�ķ���ʽΪNaClO+2NH3=N2H4+NaCl+H2O��
������ҵ�ǹ��ҹ�ҵ�Ļ�������ش������ʴ�����������е��й����⣮
��1�����ڳ�ʪ�Ŀ��������ױ���ʴ��д���������绯ѧ��ʴʱ�����ĵ缫��ӦʽFe-2e-=Fe2+��
��2��ͼ1��ʵ�����о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ��ͼ2��A��B��C��D�ĸ�������������������B������ĸ����
��3�����и���װ������������ʴ���ѵ���˳����BCA������ĸ��

���� ��1�������ºͷ�Ӧ�ȵĹ�ϵ�������ȼ���ȣ���д������Ӧ���Ȼ�ѧ����ʽ��
��2����ԭ���غ�͵����غ���д��ѧ����ʽ��
��1���������绯ѧ��ʴʱ��������Feʧ���ӷ���������Ӧ�����������ӣ�
��2�����Ի������������£�������������ʴ�����Ӵ�������ˮʱ��ʴ�����أ�
��3����ԭ��ظ�������������Ľ������ٱ���ʴ����ԭ�����������������Ľ�����������
��� �⣺��1����16g�µ����ʵ���Ϊ0.5mol��0.5molN2H4����������ȫȼ�����ɵ����ų�����312kJ���������Ȼ�ѧ��Ӧ����ʽΪ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��
�ʴ�Ϊ��N2H4��l��+O2��g��=N2��g��+2H2O��l����H=-624KJ/mol��
��2��Cl�ڷ�Ӧ����Ϊ+1�ۣ���Ӧ��Ϊ-1�ۣ�1molNaClO��Ӧת��2mol���ӣ�N�ڷ�Ӧ����Ϊ-3�ۣ���Ӧ��Ϊ-2�ۣ�����NaClO��NH3�����ʵ���֮��ΪΪ1��2���ٽ��ԭ���غ���д��ѧ����ʽΪNaClO+2NH3=N2H4+NaCl+H2O���ʴ�Ϊ��NaClO+2NH3=N2H4+NaCl+H2O��
��1���������绯ѧ��ʴʱ��������Feʧ���ӷ���������Ӧ�����������ӣ��缫��ӦʽΪFe-2e-=Fe2+���ʴ�Ϊ��Fe-2e-=Fe2+��
��2����ˮ��Һ�����ԣ����������������ʴ�������Ӵ�������ˮʱ��ʴ�����أ�����B����ʴ�����أ��ʴ�Ϊ��B��
��3���ڽ�����ʴ�У���ʴ����˳������������������ԭ��ظ�������ѧ��ʴ����ԭ�������������������������ͼ��֪��A��Fe��������B������������C����������ѧ��ʴ����������ʴ����˳����BCA���ʴ�Ϊ��BCA��
���� ���⿼��ȼ�ϵ�غͽ����ĸ�ʴ���������ȷԭ��غ͵���ԭ���ǽⱾ��ؼ���֪��������ʴ����˳�����������ⸯʴ��������ʴ��������Ŀ�ѶȲ���

| A�� | HClʮNaOH=NaClʮH2O | B�� | ̼������ȷֽ� | ||
| C�� | þ���������� | D�� | CaOʮH2O=Ca��OH��2 |
| A�� | N2�Ľṹʽ����N��N�� | B�� | F-���ӽṹʾ��ͼ��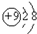 | ||
| C�� | �õ���ʽ��ʾHCl�γɹ���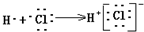 | D�� | ���������ʽ |
| A�� | NH5�ɷ��ӹ��� | B�� | NH5�������ӻ����� | ||
| C�� | NH5��N�Ļ��ϼ�Ϊ-5�� | D�� | NH5��ˮ�ķ�Ӧ�Ƿ�������ԭ��Ӧ |
| A�� | ��ͭƬ����Ƭ������һ�����ϡ�����У���Ƭ����������� | |
| B�� | ��пƬ��������Ƭ������������Ȼ�п��Һ����Ƭ�������һ��п | |
| C�� | ��ͭƬ�������Ȼ�����Һ�У���ͭƬ�������һ���� | |
| D�� | ������п������ʢ�������������Թ��У��Ӽ����Ȼ�ͭ��Һ�����ݷų����ʼӿ죬������������������ |
| A�� | 0.1mol•L-1Fe��NO3��2��Һ��Mg2+��H+��SO${\;}_{4}^{2-}$��Cl- | |
| B�� | �����£�$\frac{c��{H}^{+}��}{c��O{H}^{-}��}$=1��10-12����Һ��K+��AlO${\;}_{2}^{-}$��CO${\;}_{3}^{2-}$��Na+ | |
| C�� | Na2O2����ˮ����O2��2O22-+2H2O�TO2��+4OH- | |
| D�� | �ù���������ữ�����ҽ���Һ����ȡ�⣺2I-+H2O2�TI2+2OH- |
| A�� | ��״���£�11.2 L�����������ķ���0.5NA | |
| B�� | 28 g��ϩ�������õ��Ӷ���ĿΪ4NA | |
| C�� | ��״���£�11.2 L���ȼ�������������Ϊ0.5 NA | |
| D�� | ������ϩ����ϩ����ϩ�Ļ�����干14 g����ԭ����Ϊ3 NA |


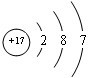 ��
��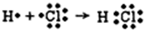 ��
��