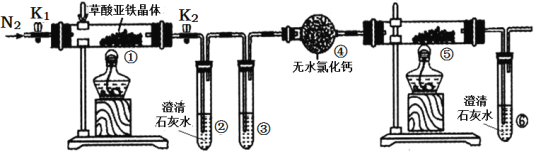
��Ŀ����
����Ŀ������ƽ�����ʢ��Һ̬��(N2H4)������������0.2molҺ̬�º�����������Ӧ�����ɵ�����ˮ�������ų�128.8kJ������(�൱��25�桢101kPa�²�õ�����)��
(1)��Ӧ���Ȼ�ѧ����ʽΪ_____________________________________________��
(2)����֪H2O(l) = H2O(g)��H= +44kJ/mol����16gҺ̬������������Һ̬ˮʱ�ų���������____kJ��
(3)�˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ���__________________________________��
���𰸡�N2H4(l)+O2(g)=N2(g)+2H2O(g) ��H=-644.0 KJ/mol 366kJ ����Ⱦ��������ɫ������
��������
(1)���ݷ�Ӧ�����������ƽ��д��ѧ����ʽ�����ݶ��ɹ�ϵ�жϣ�0.2molҺ̬����ȫ��Ӧ���ų�128.8kJ������������1molҺ̬����ȫ��Ӧ�ų�644.0 kJ��������
(2)H2O(l)=H2O(g)��H=+44kJ/mol�����ݸ�˹���ɼ�������õ���
(3)���ݲ����ж�������������Ⱦ��
(1)��Ӧ����ʽΪ��N2H4+O2=N2+2H2O��0.2molҺ̬�·ų�128.8kJ����������1molҺ̬�·ų�������Ϊ![]() =644.0 kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4(l)+O2(g)=N2(g)+2H2O(g) ��H=-644.0 KJ/mol��
=644.0 kJ�����Է�Ӧ���Ȼ�ѧ����ʽΪ��N2H4(l)+O2(g)=N2(g)+2H2O(g) ��H=-644.0 KJ/mol��
(2)��N2H4(l)+O2(g)=N2(g)+2H2O(g) ��H=-644.0 KJ/mol����H2O(l)=H2O(g)��H=+44kJ/mol��
���ݸ�˹���ɢ�-����2�õ�N2H4(l)+O2(g)=N2(g)+2H2O(l)��H=-732.0kJ/mol�� 16gҺ̬�����ʵ���=![]() =0.5mol������16gҺ̬����������Ӧ����Һ̬ˮʱ�ų���������366kJ��
=0.5mol������16gҺ̬����������Ӧ����Һ̬ˮʱ�ų���������366kJ��
(3)�˷�Ӧ���ڻ���ƽ������ͷŴ����ȺͿ��ٲ������������⣬����һ���ܴ���ŵ��Dz���Ϊ������ˮ�������ǿ����ɷֲ�����ɻ�����Ⱦ����ɫ������

 ���ʿ��ÿ��ֳɳ�ϵ�д�
���ʿ��ÿ��ֳɳ�ϵ�д�����Ŀ����25��ʱ���ܱ�������X��Y��Z��������ij�ʼŨ�Ⱥ�ƽ��Ũ�����±���
���� | X | Y | Z |
��ʼŨ��/mol��L-1 | 0.1 | 0.2 | 0 |
ƽ��Ũ��/mol��L-1 | 0.05 | 0.05 | 0.1 |
����˵��������ǣ�
A. ��Ӧ�ﵽƽ��ʱ��X��ת����Ϊ50�� B. ��Ӧ�ɱ�ʾΪX+3Y![]() 2Z����ƽ�ⳣ��Ϊ1600 C. ����ѹǿʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������ D. �ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ��
2Z����ƽ�ⳣ��Ϊ1600 C. ����ѹǿʹƽ��������Z�ķ����ƶ���ƽ�ⳣ������ D. �ı��¶ȿ��Ըı�˷�Ӧ��ƽ�ⳣ��