��Ŀ����
����Ŀ��ijͬѧ����ͼ��ʾװ�ü��������������(FeC2O4��2H2O������ɫ)���ȷֽ�IJ��ֲ������˵����ȷ����( )
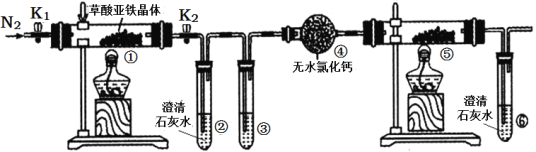
A. ͨ��N2����ҪĿ���Ƿ�ֹ�����е�ˮ�����Բ���������Ӱ��
B. ���ۺ͢��зֱ�ʢ������NaOH��Һ��CuO���壬�ɼ������ɵ�CO
C. ʵ��������е���ɫ��ĩ��ȫ��ɺ�ɫ�������һ��Ϊ��
D. �������е���ˮCaC12������ˮ����ͭ�ɼ���ֽ����ɵ�ˮ����
���𰸡�B
��������
ͨ��N2����ҪĿ���Ƿ�ֹ�����ж�����̼�������ȶԲ���������Ӱ�죬A�����������ڢ���ȥ������̼�����е���ˮ�Ȼ��ƽ���������������еĺ�ɫ������ͭ�����죬���г����ʯ��ˮ����ǣ�˵����һ����̼������B��ȷ��ʵ��������е���ɫ��ĩ��ȫ��ɺ�ɫ����������Ϊ������������������������������Щ���ʶ��Ǻ�ɫ�ģ�C�������������徭�����������ʯ��ˮ��������������Һ�����Խ����е���ˮ�Ȼ��ƻ�����ˮ����ͭ�Ͳ��ܼ���ֽ����ɵ�ˮ������D��������ȷѡ��B��

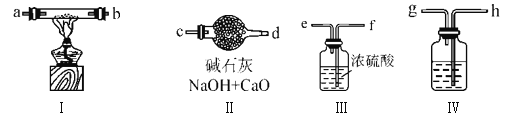
����Ŀ��ij��ѧ��ȤС����������ͼװ���Ʊ��������������۲�����ɫ��

�ṩ��ѧҩƷ����м��ϡ���ᡢ����������Һ��
��1��ϡ����Ӧ����_____________��(��д��������)��
��2����ʵ��ͨ������A��B��C�������أ��ɽ������еĿ����ž����������Ϊ���رտ���_______������________����Һ©������������Ӧһ��ʱ���ž��������ٹرտ���_______������_______���Ϳɹ۲쵽��������������ɫ���Է���ʵ�鿪ʼʱ���ž�װ���п���������_______________________________���û�ѧ��Ӧ����ʽ���𣩡�
��3��ijͬѧ��Ϊ��ʵ����Ӧ���������������ۣ�����������________________________��
��4����FeSO4��Һ�м���(NH4)2SO4������Ʊ�Ħ���ξ���[(NH4)2SO4��FeSO4��6H2O] (��Է�������392)���þ����һ���������ȶ������ױ�������������ˮ���������Ҵ���
�ٴ�Ħ����ˮ��Һ����ȡ (NH4)2SO4��FeSO4��6H2O����ľ��������______________�����Ҵ�ϴ�ӣ����
��Ϊ�˲ⶨ��Ʒ�Ĵ��ȣ���ȡ10 g��Ʒ����50 mLˮ�У����Ƴ�250 mL��Һ����Ũ��Ϊ0.01 mol��L-1������KMnO4��Һ�ζ���ÿ����ȡ����Һ�����Ϊ25.00 mL��ʵ������¼���£�
ʵ����� | ��һ�� | �ڶ��� | ������ |
���ĸ��������Һ���/mL | 25.52 | 25.02 | 24.98 |
ͨ��ʵ�����ݼ���ĸò�Ʒ����Ϊ________���ϱ��е�һ��ʵ���м�¼�������Դ��ں����Σ���ԭ�������________��
A����һ�εζ�ǰ�ζ��ܼ��������ݣ��ζ�����������
B����һ��ʵ�����ʱ���ӿ̶��߶�ȡ���Ը��������Һ�����
C����һ�εζ��õ���ƿ�ô���Һ��ϴ��
D����һ��ʵ��װ������صĵζ�����ϴ��û����ϴ