��Ŀ����
���й�����Һ��˵����ȷ���ǣ�������
| A��1L 0.2mol/L CaCl2��Һ�У�Cl-��ĿԼΪ0.2��6.02��1023�� |
| B��50mL 1mol/L AlCl3��Һ��100mL 3mol/L KClO3��Һ�е�Cl-���ʵ���Ũ����� |
| C��0.5L 1mol/L MgCl2��Һ��0.2L 1mol/L KCl��Һ�е�Cl-����Ŀ֮��Ϊ5��2 |
| D����100mL Na2SO4��KNO3�Ļ����Һ�У�Na+�����ʵ���Ũ����SO42-�����ʵ���Ũ��֮��Ϊ2��1 |
���㣺���ʵ���Ũ��,���ʵ�������ؼ���
ר�⣺���ʵ���Ũ�Ⱥ��ܽ��ר��
������A����Һ�������ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�������Ӹ������������Һ��Cl-�����ʵ���Ũ�ȣ��ٸ���n=cV�������Һ��Cl-�����ʵ����Լ���Ŀ��
B����Һ�������ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�������Ӹ�����KClO3��Һ�в�����Cl-��
C����Һ�������ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�������Ӹ������������Һ��Cl-�����ʵ���Ũ�ȣ��ٸ���n=cV�������Һ��Cl-�����ʵ����Լ���Ŀ��
D����Һ�����ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�����Ӹ�����Ȼ�������ֵ��
B����Һ�������ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�������Ӹ�����KClO3��Һ�в�����Cl-��
C����Һ�������ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�������Ӹ������������Һ��Cl-�����ʵ���Ũ�ȣ��ٸ���n=cV�������Һ��Cl-�����ʵ����Լ���Ŀ��
D����Һ�����ӵ����ʵ���Ũ��=�ε�Ũ�ȡ���ѧʽ�����Ӹ�����Ȼ�������ֵ��
���
�⣺A��1L 0.2mol/L CaCl2��Һ��Cl-�����ʵ���Ũ��Ϊ 0.2mol/L��2=0.4mol/L������Cl-�����ʵ���=1.0L��0.4mol/L=0.4mol��Cl-��ĿԼΪ0.4��6.02��1023������A����
B��50mL 1mol/L AlCl3��Һ��Cl-�����ʵ���Ũ��Ϊ 1mol/L��3=3mol/L��KClO3��Һ�в�����Cl-��Ũ��Ϊ0����B����
C��0.5L 1mol/L MgCl2��Һ��Cl-�����ʵ���Ũ��Ϊ1mol/L��2=2mol/L������Cl-�����ʵ���=0.5L��2mol/L=1mol��Cl-��ĿԼΪ6.02��1023����0.2L 1mol/L KCl��Cl-�����ʵ���Ũ��Ϊ1mol/L������Cl-�����ʵ���=0.2L��1mol/L=0.2mol��Cl-��ĿԼΪ0.2��6.02��1023����Cl-����Ŀ֮��Ϊ5��1����C����
D����100mL Na2SO4��KNO3�Ļ����Һ�У�Na+�����ʵ���Ũ��Ϊ2C��Na2SO4����SO42-�����ʵ���Ũ��ΪC��Na2SO4����Ũ��֮��Ϊ2��1����D��ȷ��
��ѡD��
B��50mL 1mol/L AlCl3��Һ��Cl-�����ʵ���Ũ��Ϊ 1mol/L��3=3mol/L��KClO3��Һ�в�����Cl-��Ũ��Ϊ0����B����
C��0.5L 1mol/L MgCl2��Һ��Cl-�����ʵ���Ũ��Ϊ1mol/L��2=2mol/L������Cl-�����ʵ���=0.5L��2mol/L=1mol��Cl-��ĿԼΪ6.02��1023����0.2L 1mol/L KCl��Cl-�����ʵ���Ũ��Ϊ1mol/L������Cl-�����ʵ���=0.2L��1mol/L=0.2mol��Cl-��ĿԼΪ0.2��6.02��1023����Cl-����Ŀ֮��Ϊ5��1����C����
D����100mL Na2SO4��KNO3�Ļ����Һ�У�Na+�����ʵ���Ũ��Ϊ2C��Na2SO4����SO42-�����ʵ���Ũ��ΪC��Na2SO4����Ũ��֮��Ϊ2��1����D��ȷ��
��ѡD��
���������⿼�����ʵ�������ؼ��㣬�Ƚϻ�������Һ�������ӵ����ʵ���Ũ��Ϊ�ε�Ũ���뻯ѧʽ�����Ӹ����Ļ�������Һ������أ�

��ϰ��ϵ�д�
�����Ŀ
���е��뷽��ʽ��д��ȷ���ǣ�������
| A����CaO����ˮ�У�CaO�TCa2++O2- |
| B����NaHSO4����ˮ�У�NaHSO4�TNa++HSO42- |
| C����Al2��SO4��3����ˮ�У�Al2��SO4��3�TAl3++SO42- |
| D����NaHCO3����ˮ�У�NaHCO3�TNa++HCO3- |
��0.2mol��������ȫȼ�պ����ɵ����建��ͨ��0.5L 2mol/L��NaOH��Һ�У��������κ���ʽ�ε����ʵ���֮��Ϊ1��3����������ǣ�������
| A������ | B������ | C������ | D������ |
��֪pH=2�ĸߵ��ᣨH5IO6����Һ��pH=12��NaOH��Һ�������ϣ����û��Һ�����ԣ�0.01mol?L-1�ĵ��ᣨHIO3����Һ��pH=12��NaOH��Һ�������ϣ����û��Һ�����ԣ����жԸߵ���͵��������ǿ���ж���ȷ���ǣ�������
| A���ߵ���͵��ᶼ��ǿ�� |
| B���ߵ���͵��ᶼ������ |
| C���ߵ��������ᣬ������ǿ�� |
| D���ߵ�����ǿ�ᣬ���������� |
�����»�ѧ����Ľ����д�����ǣ�������
| A����ҵ�����Ի�ɫ�������ں�������FeCl3 |
| B���⻯����Һ�����Ի�ɫ��������I��������������I2������Һ�� |
| C��Ũ�����Ի�ɫ���������������ֽ����ɵ�NO2��Һ��Һ�� |
| D���������ڸ��������û�ԭ����ȥ�����е����� |
���ڷ�Ӧ14CuSO4+5FeS2+12H2O�T7Cu2S+5FeSO4+12H2SO4��˵�����н�����ȷ���ǣ�������
| A��FeS2���������������ǻ�ԭ�� |
| B��ֻ��CuSO4�������� |
| C������������ͱ���ԭ������������3��7 |
| D������������ͱ���ԭ������������1��1 |
���и�������ʾ��ͼһ�µ��ǣ�������
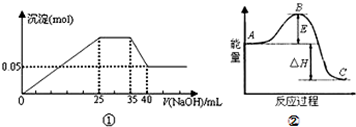

| A��ͼ�ٱ�ʾ��Mg2+��Al3+��NH4+������Һ�еμ�NaOH��Һʱ������������NaOH������Ĺ�ϵͼ�����������ӵ����ʵ���֮��Ϊ��n��Mg2+����n��Al3+����n�� NH4+��=2��1��2 |
| B��ͼ����ʹ�õ�NaOH��Ũ��Ϊ2mol?L-1 |
| C��ͼ��������A��Ӧ��������C����H��0 |
| D��ͼ�������߱�ʾij��Ӧ���̵������仯����ʹ�ô�����Eֵ���С |