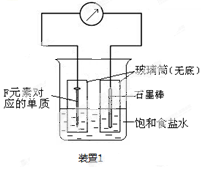
��Ŀ����
��֪XԪ��ԭ�ӵ�K��L��ĵ�����֮�ͱ�L��M��ĵ�����֮�Ͷ�1�����ӡ�YԪ�ص�ԭ���������������ڲ��������3����ZԪ�غ�����3�����Ӳ㣬�������3�����ӡ�WԪ������ϼ�����ͻ��ϼ۾���ֵ��3������������������е���������Ϊ40%��
��1��Y��W����̬�⻯����ȶ���Ϊ���û�ѧʽ��ʾ�� �� ��
��2��X�����ڿ����м������ɵĻ������� ���������ӡ����ۡ�����
��3��X��Z������������Ӧˮ���ﷴӦ�����ӷ���ʽ ��
��4��W�ĵͼ���������Y���ʵ�ˮ��Һ��Ӧ�Ļ�ѧ����ʽ ��
��5��Y��Z�γɻ�����Ļ�ѧʽ�� ��ʵ���õ��˻����ﴦ�ڹ�̬��Һ̬ʱ�����磬����ˮ�ܵ��硣�ɴ��жϸû�������� ��������ӡ����ۡ�����
��1��HCl H2S ��2������
��3��Al��OH��3+OH-=AlO2-+2H2O
��4��SO2+Cl2+2H2O=H2SO4+2HCl
��5��AlCl3 ����
����

�����ʽṹ�����ʡ�
����Ԫ�ؾ��н϶�Ŀչ�������Ե������ڵ�Cr��Fe��Co��Ni��Cu��Zn�ȶ��ֽ������γ�����
��1����̬Cuԭ�ӵĺ�������Ų�ʽΪ ��
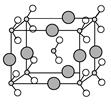
��2����ѧ��ͨ��X���߲�õ����ṹʾ��ͼ�ɼ�ʾ���£�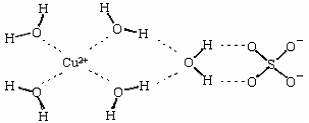
ͼ�����߱�ʾ��������Ϊ ��
��3��������Һ�백ˮ��һ�������¿�������Cu(NH3)4SO4��H2O���塣��Cu(NH3)4SO4��H2O�����У�[Cu(NH3)4]2+Ϊƽ�������νṹ�������������ṹ��ԭ������ ��������ԭ�ӵ��ӻ���������� ��
��4������������CO�����������ȣ�������ɫ�ӷ���Һ̬Ni(CO)4�����������幹�͡����Ʋ����ʻ����ľ����������� �� Ni(CO)4���������� ��
| A��ˮ | B�����Ȼ�̼ | C������ | D����������Һ |
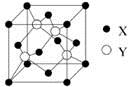
���������У��Ⱥ����Ӽ��ֺ����ۼ�����
| A��HCl | B��NaOH | C��NaCl | D��O2 |
���������У�ֻ�������Ӽ��������й��ۼ�����
| A��HCl | B��KOH | C��CaCl2 | D��CO2 |