��Ŀ����
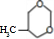
19��M��һ����Ҫ�ĺϳɲ��ϣ���ṹ��ʽΪ��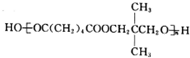
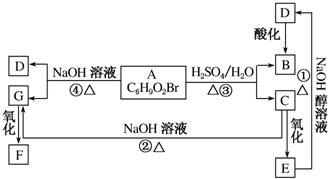
M�ĺϳ�·����ͼ�����A-F�ֱ����һ���л��
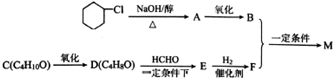
��֪��
��D�ܷ���������Ӧ�ҷ�������֧����
��
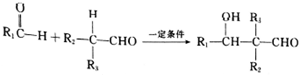
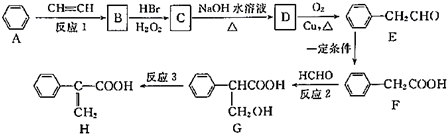
��1��E�к��й�����������ȩ�����ǻ���C������ȥ��Ӧ�����л���G��G�����ƣ�ϵͳ��������2-����ϩ��
��2��A������ˮ��ɫ���䷴Ӧ�Ļ�ѧ����ʽΪ
 +Br2��
+Br2��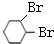 ��
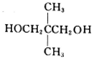
����3��D�Ľṹ��ʽ�ǣ�CH3��2CHCHO��
��4����B��F�ϳ�M�Ļ�ѧ����ʽ��n HOOC��CH2��4COOH+n
 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$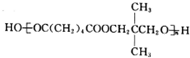 +��2n-1��H2O��
+��2n-1��H2O����5��д�������к�1����Ԫ�����˴Ź������������ַ��E��ͬ���칹��Ľṹ��ʽΪ
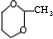 ��
�� ��
����6������˵����ȷ����acd����ѡ��������ĸ�ı�ţ�
a��A��D����ʹ��ˮ��ɫ�� b��F����Na��Ӧ����������HBr��Ӧ��
c��B��E����������Cu��OH��2��Ӧ�� d��M�̶��۷е㣮
���� 1-�Ȼ����鷢����ȥ��Ӧ����AΪ ��A����������Ӧ����B�����M�Ľṹ��ʽ��֪��B��F�������۷�Ӧ�õ�M������֪BΪHOOC��CH2��4COOH��FΪ
��A����������Ӧ����B�����M�Ľṹ��ʽ��֪��B��F�������۷�Ӧ�õ�M������֪BΪHOOC��CH2��4COOH��FΪ ��D��HCHO������Ϣ��ӳɷ�Ӧ����F��D�ܷ���������Ӧ�ҷ�������֧������DΪ��CH3��2CHCHO��CΪ��CH3��2CHCH2OH��EΪ
��D��HCHO������Ϣ��ӳɷ�Ӧ����F��D�ܷ���������Ӧ�ҷ�������֧������DΪ��CH3��2CHCHO��CΪ��CH3��2CHCH2OH��EΪ ���ݴ˽��
���ݴ˽��
��� �⣺��Ӧ�õ�M������֪BΪHOOC��CH2��4COOH��FΪ ��D��HCHO������Ϣ��ӳɷ�Ӧ����F��D�ܷ���������Ӧ�ҷ�������֧������DΪ��CH3��2CHCHO��CΪ��CH3��2CHCH2OH��EΪ
��D��HCHO������Ϣ��ӳɷ�Ӧ����F��D�ܷ���������Ӧ�ҷ�������֧������DΪ��CH3��2CHCHO��CΪ��CH3��2CHCH2OH��EΪ ��
��
��1��EΪ �����еĹ�����Ϊ��ȩ�����ǻ���CΪ��CH3��2CHCH2OH��������ȥ��Ӧ�����л���GΪCΪ��CH3��2C=CH2��G�����ƣ�ϵͳ�������ǣ�2-����ϩ��
�����еĹ�����Ϊ��ȩ�����ǻ���CΪ��CH3��2CHCH2OH��������ȥ��Ӧ�����л���GΪCΪ��CH3��2C=CH2��G�����ƣ�ϵͳ�������ǣ�2-����ϩ��
�ʴ�Ϊ��ȩ�����ǻ���2-����ϩ��
��2��AΪ �����巢���ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ
�����巢���ӳɷ�Ӧ����Ӧ�Ļ�ѧ����ʽΪ +Br2��
+Br2�� ��
��
�ʴ�Ϊ�� +Br2��
+Br2�� ��
��
��3��D�Ľṹ��ʽ�ǣ�CH3��2CHCHO���ʴ�Ϊ����CH3��2CHCHO��
��4����B��F�ϳ�M�Ļ�ѧ����ʽ�ǣ�n HOOC��CH2��4COOH+n $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$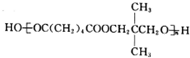 +��2n-1��H2O��
+��2n-1��H2O��
�ʴ�Ϊ��n HOOC��CH2��4COOH+n $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$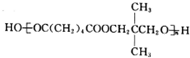 +��2n-1��H2O��
+��2n-1��H2O��
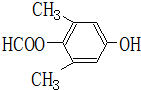
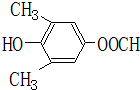
��5��EΪ ����ͬ���칹����ϣ������к�1����Ԫ�����˴Ź������������ַ壬��E��ͬ���칹��Ľṹ��ʽΪ
����ͬ���칹����ϣ������к�1����Ԫ�����˴Ź������������ַ壬��E��ͬ���칹��Ľṹ��ʽΪ  ��
�� ���ʴ�Ϊ��
���ʴ�Ϊ�� ��
�� ��
��
��6��a��A����̼̼˫���������巢���ӳɷ�Ӧ��G����ȩ�����ܱ������������߾���ʹ��ˮ��ɫ����a��ȷ��
b��F���д��ǻ�������Na��Ӧ������������������HBr����ȡ����Ӧ����b����
c��B�ͺ����Ȼ���E����ȩ��������������Cu��OH��2��Ӧ����c��ȷ��
d��MΪ�߷��ӻ����nֵ��ȷ�������ڻ����û�й̶����۷е㣬��d��ȷ��
��ѡ��acd��
���� ���⿼���л����ƶϣ��������M�Ľṹ����Ӧ��Ϣ�����ƶϣ���Ҫѧ���������չ����ŵ�������ת������5����ͬ���칹����дΪ�״��㡢�ѵ㣬ѧ���������ӻ��ṹ��

 �����������Ů��ͯ������ϵ�д�
�����������Ů��ͯ������ϵ�д�| A�� | ��ҵ������������������ϩΪԭ�ϲ����ȴ��Ҵ�����ʯ�ͻ�����������������ԭ�������ʵ� | |
| B�� | �ö������Ȼ��������������ˮ������������������ˮ����Щ | |
| C�� | ��Ϊ�������ʹ������˹�����ɸ��һ�ָ߷����л������� | |
| D�� | ԭú��������úҺ�����������Ƿ�ֹ�ҹ������ж�����������������������Ч��ʩ֮һ |
| A�� | ��������˥�߲��˽��С�ѪҺ���� | |
| B�� | ���ȵĴ�����Һ����;���մ�е����� | |
| C�� | ��������������������Ũ���� | |
| D�� | ��ʯ�ͽ��з����ѻ�������� |
| A�� | ͨ�����ĵ缫Ϊ��ص�������ͨ�������ĵ缫Ϊ���� | |
| B�� | �ڱ�״���£�ÿ����5.6 L O2���������ṩ8.3��104 C�ĵ��� | |
| C�� | ͨ�����缫�ĵ缫��ӦΪ��CH4+10OH--8e-�TCO32-+7H2O | |
| D�� | �ŵ�һ��ʱ�����ҺpH���� |
| A�� | 2NaBr+Cl2�T2NaCl+Br2 | |
| B�� | AlCl3+3NaAlO2+6H2O�T4Al��OH��3��+3NaCl | |
| C�� | 3S+6KOH�T2K2S+K2SO3+3H2O | |
| D�� | NH4Cl+NaNO2�TNaCl+N2��+2H2O |
| A�� | �Ȼ�����ӵĵ���ʽ�� | B�� | �����ӵĽṹʾ��ͼ�� | ||
| C�� | ��ϩ���ӵĽṹʽ��CH2=CH2 | D�� | �����ӵı���ģ�ͣ� |
 ��
�� ��
�� ��
�� ��
��