��Ŀ����
16���л���A��B��C��D��E��F��G���ϵ��ͼ��ʾ��5.2gF����100mL 1mol•L-1 NaOH��Һǡ����ȫ�кͣ�0.1mol F���������������Ʒ�Ӧ���ڱ�״���·���2.24L H2��D�ķ���ʽC3H3O2Na��E�ķ����к����Ȼ�����1��д���������ʵĽṹ��ʽ��GHOCH2CH2CH2OH��FHOOCCH2COOH��
��2����ѧ��Ӧ���ͣ�����ȥ��Ӧ����ˮ�ⷴӦ��
��3����ѧ����ʽ����BrCH2CH2COOH+2NaOH$��_{��}^{��}$CH2=CHCOONa+NaBr+2H2O
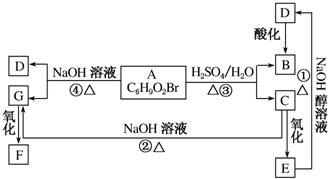
���� A�ķ���ʽΪC6H9O2Br�����ܷ���ˮ�ⷴӦ��˵��A���������������Ͷ�Ϊ$\frac{2��6+2-9-1}{2}$=2��˵��A�л�����̼̼˫����A��Ũ���������µõ�C��B��C��������E��E���������ƴ���Һ����������������D��D�ữ����B����E�ķ����к����Ȼ���D�ķ���ʽC3H3O2Na����E�к���-Br��-COOH��C����-OH��B�к���-COOH��̼̼˫������B��C������ͬ��̼ԭ����ĿΪ3����BΪCH2=CHCOOH��DΪCH2=CHCOONa��
A���������Ƶ�ˮ��Һ�����·���ˮ�ⷴӦ����D��G��G�к����ǻ���Cˮ��Ҳ�õ�G����G�к���2��-OH������ʽΪC3H8O2��G��������F��F�ܺ�NaOH��Һ��Ӧ��FΪ���ᣬ���F�Ƕ�Ԫ���ᣬ��F�ṹ��ʽΪHOOCCH2COOH���к�0.1molNaOH��ҪFΪ0.05mol������Ϊ0.05mol��104g•mol-1=5.2g������FΪHOOCCH2COOH��GΪHOCH2CH2CH2OH��CΪBrCH2CH2CH2OH��EΪBrCH2CH2COOH��AΪCH2=CHCOOCH2CH2CH2Br���ݴ˽��
��� �⣺A�ķ���ʽΪC6H9O2Br�����ܷ���ˮ�ⷴӦ��˵��A���������������Ͷ�Ϊ$\frac{2��6+2-9-1}{2}$=2��˵��A�л�����̼̼˫����A��Ũ���������µõ�C��B��C��������E��E���������ƴ���Һ����������������D��D�ữ����B����E�ķ����к����Ȼ���D�ķ���ʽC3H3O2Na����E�к���-Br��-COOH��C����-OH��B�к���-COOH��̼̼˫������B��C������ͬ��̼ԭ����ĿΪ3����BΪCH2=CHCOOH��DΪCH2=CHCOONa��
A���������Ƶ�ˮ��Һ�����·���ˮ�ⷴӦ����D��G��G�к����ǻ���Cˮ��Ҳ�õ�G����G�к���2��-OH������ʽΪC3H8O2��G��������F��F�ܺ�NaOH��Һ��Ӧ��FΪ���ᣬ���F�Ƕ�Ԫ���ᣬ��F�ṹ��ʽΪHOOCCH2COOH���к�0.1molNaOH��ҪFΪ0.05mol������Ϊ0.05mol��104g•mol-1=5.2g������FΪHOOCCH2COOH��GΪHOCH2CH2CH2OH��CΪBrCH2CH2CH2OH��EΪBrCH2CH2COOH��AΪCH2=CHCOOCH2CH2CH2Br��
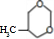
��1��������������֪��G�Ľṹ��ʽΪHOCH2CH2CH2OH��F�Ľṹ��ʽΪ��
�ʴ�Ϊ��HOCH2CH2CH2OH��HOOCCH2COOH��
��2����Ӧ����BrCH2CH2COOH������ȥ��Ӧ����Ӧ����A����ˮ�ⷴӦ��
�ʴ�Ϊ����ȥ��Ӧ��ˮ�ⷴӦ��
��3����Ӧ�ٵĻ�ѧ����ʽΪ��BrCH2CH2COOH+2NaOH$��_{��}^{��}$CH2=CHCOONa+NaBr+2H2O��
�ʴ�Ϊ��BrCH2CH2COOH+2NaOH$��_{��}^{��}$CH2=CHCOONa+NaBr+2H2O��
���� ���⿼���л�����ƶϣ�����ת����ϵ�����A�ķ���ʽ�ж�A���й����ţ������ƶϸ����ʺ��еĹ����ţ���Ҫѧ���������չ����ŵ�������ת�����Ƕ��л���֪ʶ���ۺ����ü�ѧ��˼ά�����Ŀ��飬�Ѷ��еȣ�

 ��У����ϵ�д�
��У����ϵ�д�| A�� | 4�� | B�� | 3 �� | C�� | 2�� | D�� | 1�� |
| A�� | 16g��CH4���4NA��C-H�� | |
| B�� | 1mol•L-1 NaCl��Һ�к���NA��Na+ | |
| C�� | ���³�ѹ�£�22.4L CO2�к���NA��CO2���� | |
| D�� | ��״���£�33.6L�������к��з�ԭ�ӵ���ĿΪ1.5NA |
| A�� | 0.1 mol•L-1Na2S2O3��H2SO4��5mL����ˮ5mL����Ӧ�¶�10�� | |
| B�� | 0.1 mol•L-1Na2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�10�� | |
| C�� | 0.1 mol•L-1Na2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�30�� | |
| D�� | 0.2 mol•L-1Na2S2O3��H2SO4��5mL����ˮ10mL����Ӧ�¶�30�� |
| A�� | �����ʵ���Ũ�ȵİ�ˮ������������ϣ�c��Cl-����c��NH4+����c��H+����c��OH-�� | |
| B�� | c��NH4+����ȣ�NH4��2SO4��Һ����NH4��2CO3��Һ��NH4Cl��Һ��c[��NH4��2CO3]��c[��NH4��2SO4]��c[NH4Cl] | |
| C�� | ���ʵ���Ũ����ȵ�CH3COOH��CH3COONa��Һ�������ϣ�c��Na+��+c��OH-��=c��H+��+c��CH3COOH�� | |
| D�� | �����£�NaB��Һ��pH=8��c��Na+��-c��B-��=9.9��10-7mol•L-1 |
| A�� | ���ӵ�����ṹΪ�������� | B�� | ̼ԭ����sp3�ӻ� | ||
| C�� | ���ڼ��Է��� | D�� | �������칹�� |
| A�� | ԭ�Ӱ뾶��A��B��D��C | B�� | ԭ�ӵ�����������Ŀ��A��B��D��C | ||
| C�� | ԭ��������d��c��b��a | D�� | ���Ӱ뾶��C2-��D-��B+��A2+ |
| A�� | Y��Ũ�Ȳ���ʱ��������仯 | B�� | ��Ӧ��ϵ����ѹǿ�㶨 | ||
| C�� | c��X����c��Y��=1��3 | D�� | ÿ����3mol��Yͬʱ����lmol��X |
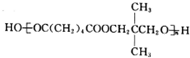
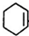 +Br2��
+Br2��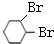 ��
��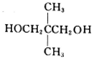 $\stackrel{һ������}{��}$
$\stackrel{һ������}{��}$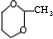 ��
�� ��
��