��Ŀ����
̼�����ʮ����Ҫ������Ԫ�أ����ʯ��SiC������ĥ����ʴ���ԣ�Ӧ�ù㷺��
��1��̼Ԫ�������ڱ��е�λ����_______________����ԭ�Ӻ���ͨ��δ�ɶԵ�����Ϊ___________����
��2����֪2Ca3(PO4)2(s)+10C(s)��P4(g)+6CaO(s)+10CO(g)��Ӧ�У����ƻ��Ļ�ѧ����________��
a�����Ӽ� b.���Թ��ۼ� c. �Ǽ��Թ��ۼ�
��3��һ�������£�Na��ԭCCl4���Ʊ����ʯ����Ӧ������ȴ�����º�ȥ�ֲ�Ʒ�������Ƶ��Լ�Ϊ_______________��
��4������������ȷ����____________����ţ���
��Na��ԭCCl4�ķ�Ӧ��Cl2��H2O�ķ�Ӧ�����û���Ӧ
��ˮ�����ɱ��ۻ�ʱ�˷����Ӽ���������������ͬ
��NaSiO3��Һ��SO3�ķ�Ӧ�������ƶ�Si��S�ķǽ�����ǿ��
��Si��һ�������¿���FeO�����û���Ӧ
��1��̼Ԫ�������ڱ��е�λ����_______________����ԭ�Ӻ���ͨ��δ�ɶԵ�����Ϊ___________����
��2����֪2Ca3(PO4)2(s)+10C(s)��P4(g)+6CaO(s)+10CO(g)��Ӧ�У����ƻ��Ļ�ѧ����________��
a�����Ӽ� b.���Թ��ۼ� c. �Ǽ��Թ��ۼ�
��3��һ�������£�Na��ԭCCl4���Ʊ����ʯ����Ӧ������ȴ�����º�ȥ�ֲ�Ʒ�������Ƶ��Լ�Ϊ_______________��
��4������������ȷ����____________����ţ���
��Na��ԭCCl4�ķ�Ӧ��Cl2��H2O�ķ�Ӧ�����û���Ӧ
��ˮ�����ɱ��ۻ�ʱ�˷����Ӽ���������������ͬ
��NaSiO3��Һ��SO3�ķ�Ӧ�������ƶ�Si��S�ķǽ�����ǿ��
��Si��һ�������¿���FeO�����û���Ӧ
(���8�֣�ÿС��2��) ��1���ڶ����ڵ�IVA�壬 2 ��2��abc ��3��ˮ�����Ҵ��� ��4���ۢ�
�����������1��̼Ԫ�صĵ��Ӳ���Ϊ2������������Ϊ4�����������ڱ��е�λ���ǵڶ����ڵ�IVA�壬̼Ԫ�ص�2p�ܼ�����2��δ�ɶԵ��ӡ�
��2����2Ca3(PO4)2��s��+10C��s����P4��g��+6CaO��s��+10CO����֪�������Ӻ����������֮������Ӽ����ƻ�����ԭ�Ӻ���ԭ�Ӽ�ļ��Թ��ۼ����ƻ�������C�зǼ��Թ��ۼ�Ҳ���ƻ�����˴�ѡabc��
��3������Na������ˮ�����Ҵ���������Ӧ�������ʯ����ˮ�����Ҵ�����Ӧ�����Գ�ȥ�ֲ�Ʒ���������ƿ���ˮ�����Ҵ�����
��4����Na��ԭCCl4����NaCl��C�������û���Ӧ����Cl2��H2O��Ӧ����HCl��HClO�������û���Ӧ���ʢٴ���ˮ������ԭ�Ӿ��壬���ɱ����ڷ��Ӿ��壬�ۻ�ʱ�˷����Ӽ������������Ͳ���ͬ��ǰ���ǹ��ۼ��������Ƿ��Ӽ����������ʢڴ���Na2SiO3��Һ��SO3�ķ�Ӧ��˵������H2SiO3��H2SO4�����������ƶ�Si��S�ķǽ�����ǿ�����ʢ���ȷ����C��Siͬ�����������ƣ�Si��һ�������¿���FeO�����û���Ӧ���ʢ���ȷ����ѡ�ۢܡ�

��ϰ��ϵ�д�
 ��������һ���þ�ϵ�д�
��������һ���þ�ϵ�д� Сѧ��10����Ӧ����ϵ�д�
Сѧ��10����Ӧ����ϵ�д�
�����Ŀ
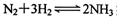 ʵ�ִ�������⡣����˵����ȷ���� ��
ʵ�ִ�������⡣����˵����ȷ���� ��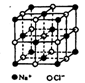

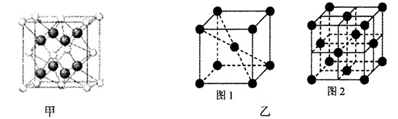
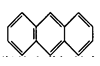 ��ƽ��ṹ)����________(����ԡ��Ǽ��ԡ�)���ӡ�
��ƽ��ṹ)����________(����ԡ��Ǽ��ԡ�)���ӡ�