��Ŀ����
̼���������ǵ����Ϸḻ��Ԫ�ء�
��1��C��N��O�ĵ�һ�������ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��_________��
��2��ǰ������Ԫ���У���̬ԭ��δ�ɶԵ������뵪��ͬ��Ԫ����_________�֡�
��3�����ж�NH3����ˮ���γ�NH3��H2O�ĺ����ṹ��_______������ĸ���ţ�������������____ ___________��
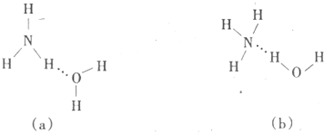
��4��H2O������H+��ϵĹ�����δ�����ı����_________������ţ���
a�����Ŀռ乹�� b��Oԭ�ӵ��ӻ���ʽ c��H��O��H�ļ���
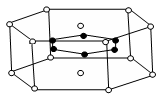
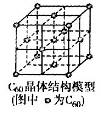
��5��C60���壨��ṹģ����ͼ����ÿ��C60������Χ������������ҵȾ����C60������__________����
��1��C��N��O�ĵ�һ�������ɴ�С��˳��Ϊ����Ԫ�ط��ű�ʾ��_________��
��2��ǰ������Ԫ���У���̬ԭ��δ�ɶԵ������뵪��ͬ��Ԫ����_________�֡�
��3�����ж�NH3����ˮ���γ�NH3��H2O�ĺ����ṹ��_______������ĸ���ţ�������������____ ___________��
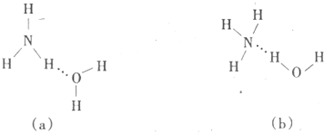
��4��H2O������H+��ϵĹ�����δ�����ı����_________������ţ���
a�����Ŀռ乹�� b��Oԭ�ӵ��ӻ���ʽ c��H��O��H�ļ���
��5��C60���壨��ṹģ����ͼ����ÿ��C60������Χ������������ҵȾ����C60������__________����

��8�֣���1��N��O��C��1�֣� ��2��4��2�֣�
��3��b��1�֣���һˮ�ϰ��������NH4����OH����1�֣� ��4��b��1�֣� ��5��12��2�֣�
��3��b��1�֣���һˮ�ϰ��������NH4����OH����1�֣� ��4��b��1�֣� ��5��12��2�֣�
�����������1��ͬ���ڵ�һ������������Ҿ����������ƣ����Ե�һ������O��C�����ڵ�Ԫ��ԭ��2p�ܼ���3�����ӣ����ڰ����ȶ�״̬�������ϵͣ���һ�����ܴ�������Ԫ�أ�����C��N��O�ĵ�һ�������ɴ�С��˳��ΪN��O��C��
��2����Ԫ�غ�������Ų���1s22s22p3�����Ի�̬ԭ��δ�ɶԵ�������3�������ǰ������Ԫ���У���̬ԭ��δ�ɶԵ������뵪��ͬ��Ԫ����P��V��Co��As��������4��Ԫ�ء�
��3����Ϊһˮ�ϰ�����Һ���ܵ������NH4����OH�����������ṹ�е�Ԫ����ˮ�е���Ԫ���γ������������ȷ�Ľṹ��b����ѡb��
��4��H2O������H+����γ�ˮ�������ӣ���ռ乹����V�α�Ϊ�����Σ�������Ŀռ乹�͡�H��O��H�ļ��Ǿ������仯����Oԭ�ӵ��ӻ���ʽ���䣬��Ȼ��sp3�ӻ�����ѡb��
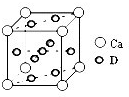
��5������C60�����ṹ��֪��ÿ��C60������Χ������������ҵȾ����C60����λ�����Ĵ���������3��8��2��12����

��ϰ��ϵ�д�
�����Ŀ

 ����
���� ���ĸ�����Ϊ ��
���ĸ�����Ϊ ��
 ������ĿΪ ��
������ĿΪ ��



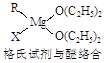
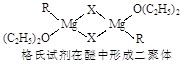
 Ti+2MgCl2
Ti+2MgCl2