��Ŀ����
X��Y��Z��W�Ƕ������г���Ԫ�أ��������Ϣ���±���
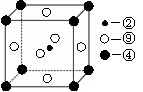
��1��WԪ��λ��Ԫ�����ڱ��е� ���� �壬��Yͬ������λ�ڵ�������Ԫ�صĻ�̬ԭ�Ӻ�������Ų�ʽΪ ��
��2��XY2�����к��еĦҼ��ͦм�������Ϊ ������ͬ������XY2��XO2��ȣ��۵�ϸߵ��� (�ѧʽ)�����������۽���Z�������Ԫ�ص�һ�����ܴ���ͬ���ں�һ��Ԫ�ص�ԭ�� ��
��3��Y��WԪ���γɵĻ�����Y2W2�����⻯��ZH3����ˮ�����·�Ӧ����Y4Z4������Y8��һ�ֿ�����Ϊ���ʵ����ʣ���д����Ӧ��ѧ����ʽ�� ��
��4����֪����lmolY(s)ת��Ϊ��̬Y(g) \��������280 kJ��
��2XO(g)+O2(g)= 2XO2(g) ��H=-566.0KJ/mol��
��Y(s)+O2(g)=YO2(g) ��H=-299.0KJ/mol��һ�������£���������X��������XO��Y��������YO2����Y(g)���ʺ�X����������ﵽ������ȾĿ�ġ���д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
| Ԫ�� | �����Ϣ |
| X | XԪ���γɵ�һ��ͬ������������Ȼ������Ӳ�����ĵ��� |
| Y | ���³�ѹ�£�Y�����ǵ���ɫ���壬��������ڻ�ҩ |
| Z | Z�Ļ�̬ԭ�Ӻ�����3���ܼ����е��ӣ�����3�������� |
| W | WԪ���γɵ�˫ԭ�ӷ��ӣ�������Ϊ����ɫ���壬һ�ֳ�����ҵԭ�� |
��1��WԪ��λ��Ԫ�����ڱ��е� ���� �壬��Yͬ������λ�ڵ�������Ԫ�صĻ�̬ԭ�Ӻ�������Ų�ʽΪ ��
��2��XY2�����к��еĦҼ��ͦм�������Ϊ ������ͬ������XY2��XO2��ȣ��۵�ϸߵ��� (�ѧʽ)�����������۽���Z�������Ԫ�ص�һ�����ܴ���ͬ���ں�һ��Ԫ�ص�ԭ�� ��
��3��Y��WԪ���γɵĻ�����Y2W2�����⻯��ZH3����ˮ�����·�Ӧ����Y4Z4������Y8��һ�ֿ�����Ϊ���ʵ����ʣ���д����Ӧ��ѧ����ʽ�� ��
��4����֪����lmolY(s)ת��Ϊ��̬Y(g) \��������280 kJ��
��2XO(g)+O2(g)= 2XO2(g) ��H=-566.0KJ/mol��
��Y(s)+O2(g)=YO2(g) ��H=-299.0KJ/mol��һ�������£���������X��������XO��Y��������YO2����Y(g)���ʺ�X����������ﵽ������ȾĿ�ġ���д���÷�Ӧ���Ȼ�ѧ����ʽ�� ��
(��13��)
(1)����V��A(2��) 1s22s22p63s23p63d104s24p4(��[Ar]3d104s24p4)(2��)
(2)1��1(1��) CS2(1��)
����NԪ����ڵĵ�VA�壬p�ܼ��ϴ��ڰ���������Ϻ��ع����а���Ϊ�ȶ��ṹ�����Ե�һ�����ܴ���ͬ���ں�һ����Ԫ��(2��)
(3)6S2Cl2+16NH3=S4N4+S8+12NH4C1(2��)[��ѧʽ����ƽ����0��]
(4)2CO(g)+SO2(g)=2CO2(g)+S(g) ��H="+10.0" KJ/mol(3��)[��ѧʽ����ƽ����0�֣�״̬����ֵ����2��]
(1)����V��A(2��) 1s22s22p63s23p63d104s24p4(��[Ar]3d104s24p4)(2��)
(2)1��1(1��) CS2(1��)
����NԪ����ڵĵ�VA�壬p�ܼ��ϴ��ڰ���������Ϻ��ع����а���Ϊ�ȶ��ṹ�����Ե�һ�����ܴ���ͬ���ں�һ����Ԫ��(2��)
(3)6S2Cl2+16NH3=S4N4+S8+12NH4C1(2��)[��ѧʽ����ƽ����0��]
(4)2CO(g)+SO2(g)=2CO2(g)+S(g) ��H="+10.0" KJ/mol(3��)[��ѧʽ����ƽ����0�֣�״̬����ֵ����2��]
�������������������Ƴ�X��Y��Z��W�ֱ�ΪC��S��N��Cl��
��1��Br�ĵ����Ų�ʽ��������ΪP������������ȫ������������д����
��2��CS2��CO2���ƣ�ΪS=C=S�ṹ�����Ӿ����۷е�Ƚϣ�����ṹ���ƣ���Է�������Խ���Ӽ�������Խ���۷е�Խ�ߣ�NΪ�����״̬���ʵ�һ�����ܸ���O��
��3�����ݷ�Ӧ��ΪS2Cl2��NH3��������ΪS4N4��S8�����������غ����д����Ӧ����ʽ��
��4��Ŀ�귴ӦΪ2CO(g)+SO2(g)=2CO2(g)+S(g)�����ݸ�˹���ɣ���Ϊ��-��+�٣��ʵõ����Ȼ�ѧ����ʽ��

��ϰ��ϵ�д�
 �������ͬ������ϵ�д�
�������ͬ������ϵ�д�
�����Ŀ
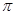 ���� mol��
���� mol��
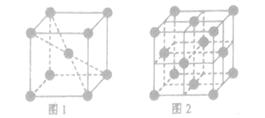
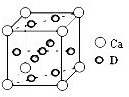
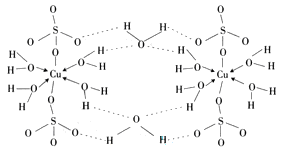
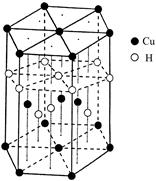
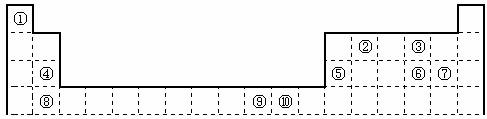
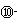 ��Ԫ�صĺ�������Ų�ʽ_______________________________________;
��Ԫ�صĺ�������Ų�ʽ_______________________________________;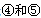 ��һ�����ܵĴ�С��ϵΪ________________(��Ԫ�ط��ű�ʾ)
��һ�����ܵĴ�С��ϵΪ________________(��Ԫ�ط��ű�ʾ)